Int J Stem Cells.
2016 Nov;9(2):250-263. 10.15283/ijsc16010.
Transplantation of Autologous Bone Marrow Mesenchymal Stem Cells into the Testes of Infertile Male Rats and New Germ Cell Formation
- Affiliations
-
- 1Infertility and Reproductive Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran. ghasemzadeh78@gmail.com m.ghasemzadeh@mubabol.ac.ir
- 2Department of Clinical Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. alibatavani@yahoo.com
- 3Department of Stem Cells and Developmental Biology, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran. eslami@royaninstitute.org
- KMID: 2362519
- DOI: http://doi.org/10.15283/ijsc16010
Abstract
- BACKGROUND
Mesenchymal stem cells (MSCs), have been suggested as a potential choice for treatment of male infertility. Yet, the effects of MSCs on regeneration of germinal epithelium of seminiferous tubules and recovery of spermatogenesis have remained controversial. In this research, we have evaluated and compared the fate of autologous bone marrow (BM)-MSCs during three different periods of time- 4, 6 and 8 weeks after transplantation into the testes of busulfan-induced infertile male rats.
METHODS
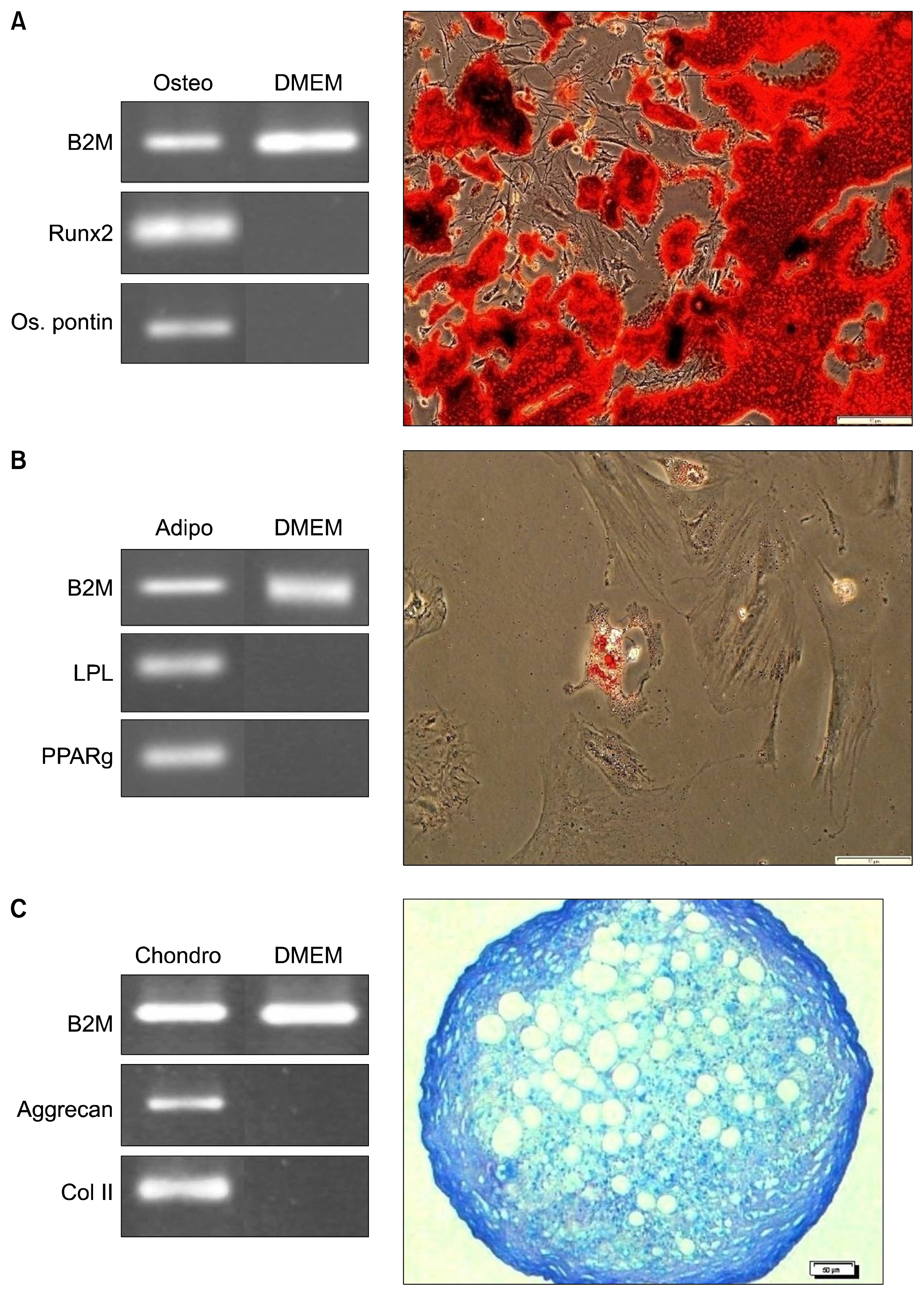
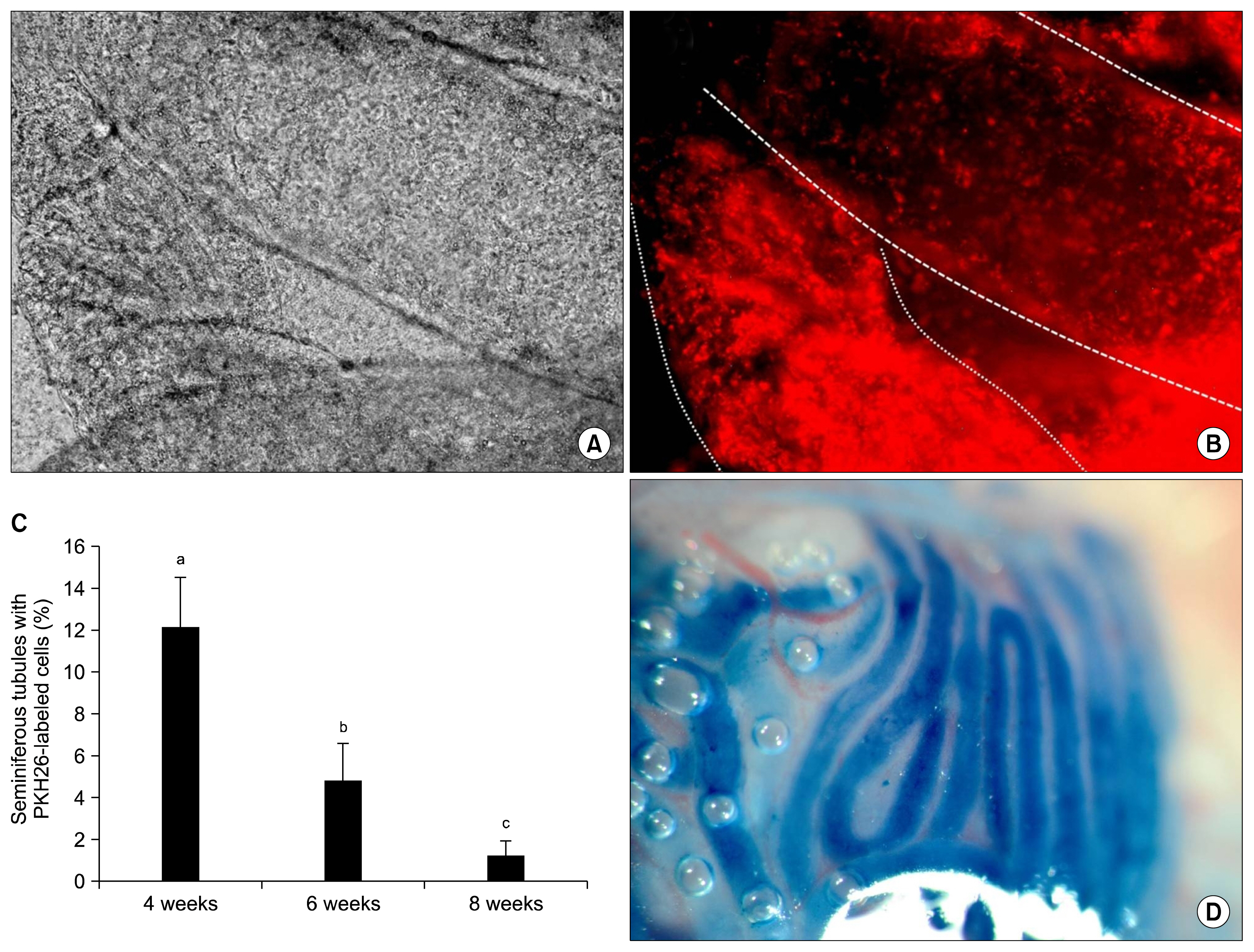
Rats BM samples were collected from tibia bone under anesthesia. The samples were directly cultured in culture medium. Isolated, characterized and purified BM-MSCs were labeled with PKH26, and transplanted into the testes of infertile rats. After 4, 6 and 8 weeks, the testes were removed and underwent histological evaluations.
RESULTS
Immunohistochemical analysis showed that transplanted BM-MSCs survived in all three groups. Some of the cells homed at the germinal epithelium and expressed spermatogonia markers (Dazl and Stella). The number of homed spermatogonia-like cells in 4-week testes, was more than the 6-week testes. The 8-week testes had the least numbers of homed cells (p<0.05). Immunostaining for vimentin showed that BM-MSCs did not differentiate into the sertoli cells in the testes.
CONCLUSIONS
From our results, it could be concluded that, autologous BM-MSCs could survive in the testis, migrate onto the seminiferous tubules basement membrane and differentiate into spermatogonia. Although, no more differentiation was observed in the produced spermatogonia, generation of such endogenous GCs would be a really promising achievement for treatment of male infertility using autologous stem cells.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Nayernia K, Li M, Jaroszynski L, Khusainov R, Wulf G, Schwandt I, Korabiowska M, Michelmann HW, Meinhardt A, Engel W. Stem cell based therapeutical approach of male infertility by teratocarcinoma derived germ cells. Hum Mol Genet. 2004; 13:1451–1460. DOI: 10.1093/hmg/ddh166. PMID: 15163638.
Article2. Madhukar D, Rajender S. Hormonal treatment of male infertility: promises and pitfalls. J Androl. 2009; 30:95–112. DOI: 10.2164/jandrol.108.005694.
Article3. Miyamoto T, Tsujimura A, Miyagawa Y, Koh E, Namiki M, Sengoku K. Male infertility and its causes in human. Adv Urol. 2012; 2012:384520. DOI: 10.1155/2012/384520.
Article4. Esteves SC, Miyaoka R, Agarwal A. Surgical treatment of male infertility in the era of intracytoplasmic sperm injection - new insights. Clinics (Sao Paulo). 2011; 66:1463–1478. DOI: 10.1590/S1807-59322011000800026.
Article5. Volarevic V, Bojic S, Nurkovic J, Volarevic A, Ljujic B, Arsenijevic N, Lako M, Stojkovic M. Stem cells as new agents for the treatment of infertility: current and future perspectives and challenges. Biomed Res Int. 2014; 2014:507234. DOI: 10.1155/2014/507234. PMID: 24826378. PMCID: 4009115.
Article6. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008; 8:726–736. DOI: 10.1038/nri2395.
Article7. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006; 119:2204–2213. DOI: 10.1242/jcs.02932. PMID: 16684817.
Article8. Pountos I, Corscadden D, Emery P, Giannoudis PV. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury. 2007; 38(Suppl 4):S23–S33. DOI: 10.1016/S0020-1383(08)70006-8.
Article9. Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001; 19:180–192. DOI: 10.1634/stemcells.19-3-180. PMID: 11359943.
Article10. Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001; 98:7841–7845. DOI: 10.1073/pnas.141221698. PMID: 11427725. PMCID: 35429.
Article11. Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011; 12:126–131. DOI: 10.1038/nrm3049. PMID: 21253000. PMCID: 3346289.
Article12. Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004; 18:980–982. PMID: 15084518.
Article13. Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, Gromoll J, Engel W. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006; 86:654–663. DOI: 10.1038/labinvest.3700429. PMID: 16652109.
Article14. Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005; 33:145–152. DOI: 10.1165/rcmb.2004-0330OC. PMID: 15891110. PMCID: 2715309.
Article15. Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol. 2008; 40:815–820. DOI: 10.1016/j.biocel.2008.01.007. PMID: 18295530.
Article16. Reinders ME, Fibbe WE, Rabelink TJ. Multipotent mesenchymal stromal cell therapy in renal disease and kidney transplantation. Nephrol Dial Transplant. 2010; 25:17–24. DOI: 10.1093/ndt/gfp552.
Article17. Farini A, Sitzia C, Erratico S, Meregalli M, Torrente Y. Clinical applications of mesenchymal stem cells in chronic diseases. Stem Cells Int. 2014; 2014:306573. DOI: 10.1155/2014/306573. PMID: 24876848. PMCID: 4021690.
Article18. Drusenheimer N, Wulf G, Nolte J, Lee JH, Dev A, Dressel R, Gromoll J, Schmidtke J, Engel W, Nayernia K. Putative human male germ cells from bone marrow stem cells. Soc Reprod Fertil Suppl. 2007; 63:69–76. PMID: 17566262.19. Ghasemzadeh-Hasankolaei M, Eslaminejad MB, Batavani R, Sedighi-Gilani M. Comparison of the efficacy of three concentrations of retinoic acid for transdifferentiation induction in sheep marrow-derived mesenchymal stem cells into male germ cells. Andrologia. 2014; 46:24–35. DOI: 10.1111/and.12037.
Article20. Ghasemzadeh-Hasankolaei M, Sedighi-Gilani MA, Eslaminejad MB. Induction of ram bone marrow mesenchymal stem cells into germ cell lineage using transforming growth factor-β superfamily growth factors. Reprod Domest Anim. 2014; 49:588–598. DOI: 10.1111/rda.12327. PMID: 24888234.
Article21. Hua J, Pan S, Yang C, Dong W, Dou Z, Sidhu KS. Derivation of male germ cell-like lineage from human fetal bone marrow stem cells. Reprod Biomed Online. 2009; 19:99–105. DOI: 10.1016/S1472-6483(10)60052-1. PMID: 19573297.
Article22. Hua J, Yu H, Dong W, Yang C, Gao Z, Lei A, Sun Y, Pan S, Wu Y, Dou Z. Characterization of mesenchymal stem cells (MSCs) from human fetal lung: potential differentiation of germ cells. Tissue Cell. 2009; 41:448–455. DOI: 10.1016/j.tice.2009.05.004. PMID: 19651422.
Article23. Huang P, Lin LM, Wu XY, Tang QL, Feng XY, Lin GY, Lin X, Wang HW, Huang TH, Ma L. Differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells into germ-like cells in vitro. J Cell Biochem. 2010; 109:747–754. PMID: 20052672.
Article24. Shirazi R, Zarnani AH, Soleimani M, Abdolvahabi MA, Nayernia K, Ragerdi Kashani I. BMP4 can generate primordial germ cells from bone-marrow-derived pluripotent stem cells. Cell Biol Int. 2012; 36:1185–1193. DOI: 10.1042/CBI20110651. PMID: 22988836.
Article25. Horn MM, Paz AH, Duarte ME, Baldo G, Belardinelli MC, Matte U, Lima EO, Passos EP. Germinative testicular cells and bone marrow mononuclear cells transplanted to a rat model of testicular degeneration. Cloning Stem Cells. 2008; 10:543–546. DOI: 10.1089/clo.2008.0004. PMID: 18795870.
Article26. Lassalle B, Mouthon MA, Riou L, Barroca V, Coureuil M, Boussin F, Testart J, Allemand I, Fouchet P. Bone marrow-derived stem cells do not reconstitute spermatogenesis in vivo. Stem Cells. 2008; 26:1385–1386. DOI: 10.1634/stemcells.2007-0767. PMID: 18339770.
Article27. Cakici C, Buyrukcu B, Duruksu G, Haliloglu AH, Aksoy A, Isık A, Uludag O, Ustun H, Subası C, Karaoz E. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: the sperm generation. Biomed Res Int. 2013; 2013:529589. DOI: 10.1155/2013/529589. PMID: 23509736. PMCID: 3590610.
Article28. Monsefi M, Fereydouni B, Rohani L, Talaei T. Mesenchymal stem cells repair germinal cells of seminiferous tubules of sterile rats. Iran J Reprod Med. 2013; 11:537–544.29. Zhang D, Liu X, Peng J, He D, Lin T, Zhu J, Li X, Zhang Y, Wei G. Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model. Int J Mol Sci. 2014; 15:13151–13165. DOI: 10.3390/ijms150813151. PMID: 25062349. PMCID: 4159785.
Article30. Tamadon A, Mehrabani D, Rahmanifar F, Jahromi AR, Panahi M, Zare S, Khodabandeh Z, Jahromi IR, Tanideh N, Dianatpour M, Ramzi M, Koohi-Hoseinabadi O. Induction of Spermatogenesis by Bone Marrow-derived Mesenchymal Stem Cells in Busulfan-induced Azoospermia in Hamster. Int J Stem Cells. 2015; 8:134–145. DOI: 10.15283/ijsc.2015.8.2.134. PMID: 26634062. PMCID: 4651278.
Article31. Ghasemzadeh-Hasankolaei M, Eslaminejad MB, Batavani R, Ghasemzadeh-Hasankolaei M. Male and female rat bone marrow-derived mesenchymal stem cells are different in terms of the expression of germ cell specific genes. Anat Sci Int. 2015; 90:187–196. DOI: 10.1007/s12565-014-0250-1.
Article32. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. DOI: 10.1080/14653240600855905. PMID: 16923606.
Article33. Ghasemzadeh-Hasankolaei M, Eslaminejad MB, Sedighi-Gilani M, Mokarizadeh A. Starvation is more efficient than the washing technique for purification of rat Sertoli cells. In Vitro Cell Dev Biol Anim. 2014; 50:723–730. DOI: 10.1007/s11626-014-9762-1. PMID: 24789729.
Article34. Ghasemzadeh-Hasankolai M, Batavani R, Eslaminejad MB, Sedighi-Gilani M. Effect of zinc ions on differentiation of bone marrow-derived mesenchymal stem cells to male germ cells and some germ cell-specific gene expression in rams. Biol Trace Elem Res. 2012; 150:137–146. DOI: 10.1007/s12011-012-9484-8. PMID: 22890879.
Article35. Ghasemzadeh-Hasankolaei M, Eslaminejad MB, Sedighi-Gilani M. Derivation of male germ cells from ram bone marrow mesenchymal stem cells by three different methods and evaluation of their fate after transplantation into the testis. In Vitro Cell Dev Biol Anim. 2016; 52:49–61. DOI: 10.1007/s11626-015-9945-4.
Article36. Herrid M, Vignarajan S, Davey R, Dobrinski I, Hill JR. Successful transplantation of bovine testicular cells to heterologous recipients. Reproduction. 2006; 132:617–624. DOI: 10.1530/rep.1.01125. PMID: 17008473.
Article37. Ogawa T, Aréchaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997; 41:111–122. PMID: 9074943.38. Zhang Z, Shao S, Shetty G, Meistrich ML. Donor Sertoli cells transplanted into irradiated rat testes stimulate partial recovery of endogenous spermatogenesis. Reproduction. 2009; 137:497–508. DOI: 10.1530/REP-08-0120.
Article39. Rodriguez-Sosa JR, Silvertown JD, Foster RA, Medin JA, Hahnel A. Transduction and transplantation of spermatogonia into the testis of ram lambs through the extra-testicular rete. Reprod Domest Anim. 2009; 44:612–620. DOI: 10.1111/j.1439-0531.2007.01030.x.
Article40. Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Natl Acad Sci USA. 2001; 98:6186–6191. DOI: 10.1073/pnas.111158198. PMID: 11371640. PMCID: 33443.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Stem Cells and Niemann Pick Disease
- Transplantation of Culture-Expanded Bone Marrow Mesenchymal Cells and Type I Collagen Gel-Xenograft Complex
- The Evolving Role of Myeloablative Chemotherapy with Stem Cell Transplantation for the Treatment of Autoimmune Disease
- Stem Cell Research in Cardiovascular System