Anat Cell Biol.
2017 Sep;50(3):219-229. 10.5115/acb.2017.50.3.219.
Protective effect of Rhus verniciflua Stokes extract in an experimental model of post-menopausal osteoporosis
- Affiliations
-
- 1Department of Anatomy, Konyang University College of Medicine, Daejeon, Korea. jjzzy@konyang.ac.kr
- 2Department of Microbiology, Konyang University College of Medicine, Daejeon, Korea.
- 3Lifetree Co., Ltd., Suwon, Korea.
- 4School of Life Sciences & Biotechnology, Korea University, Seoul, Korea.
- 5Myunggok Research Institute, Konyang University College of Medicine, Daejeon, Korea.
- KMID: 2390484
- DOI: http://doi.org/10.5115/acb.2017.50.3.219
Abstract
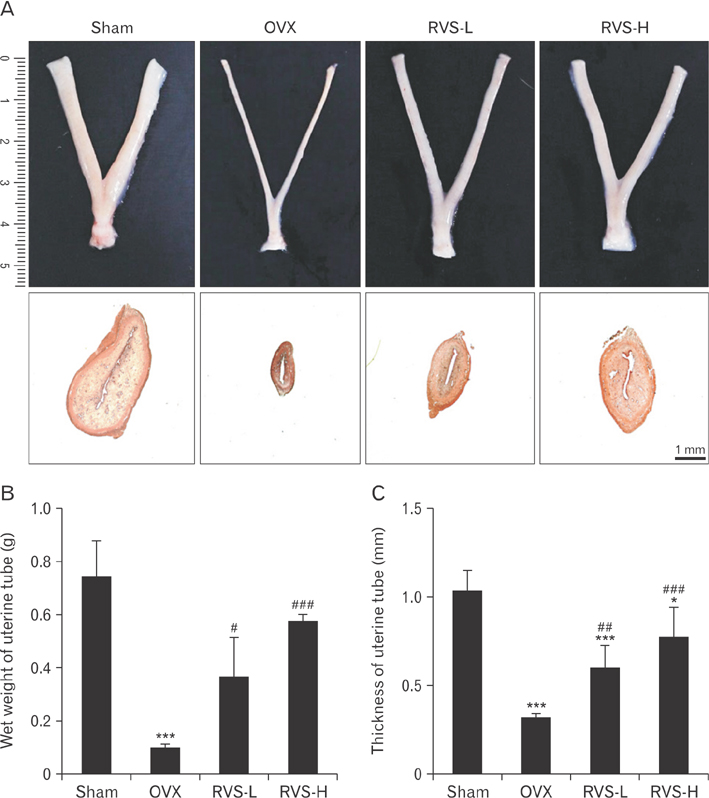
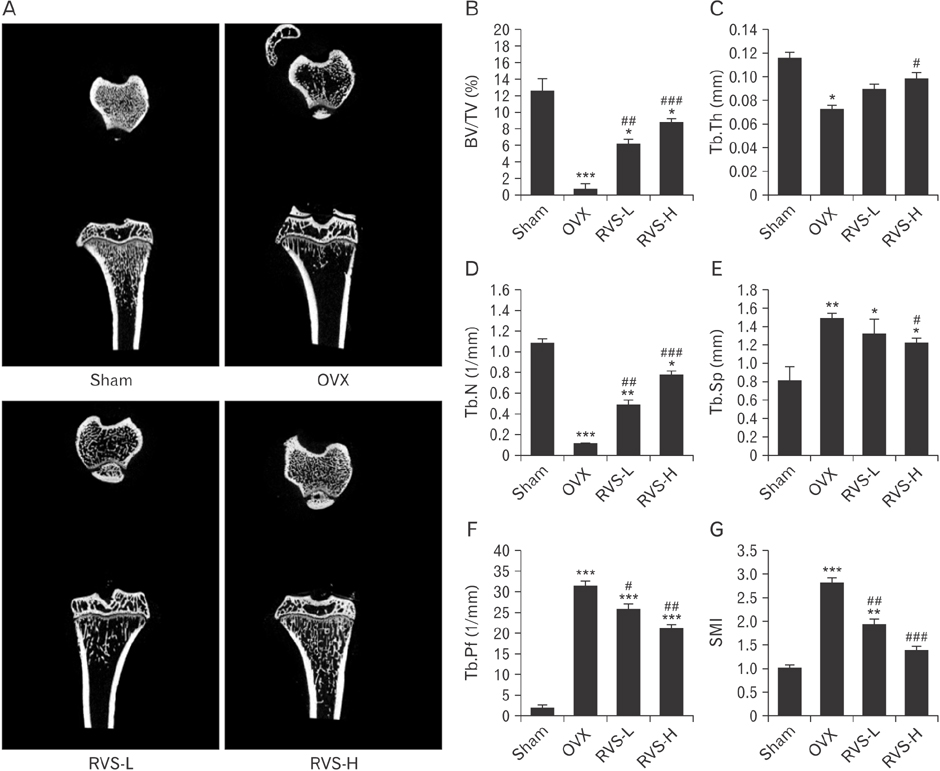
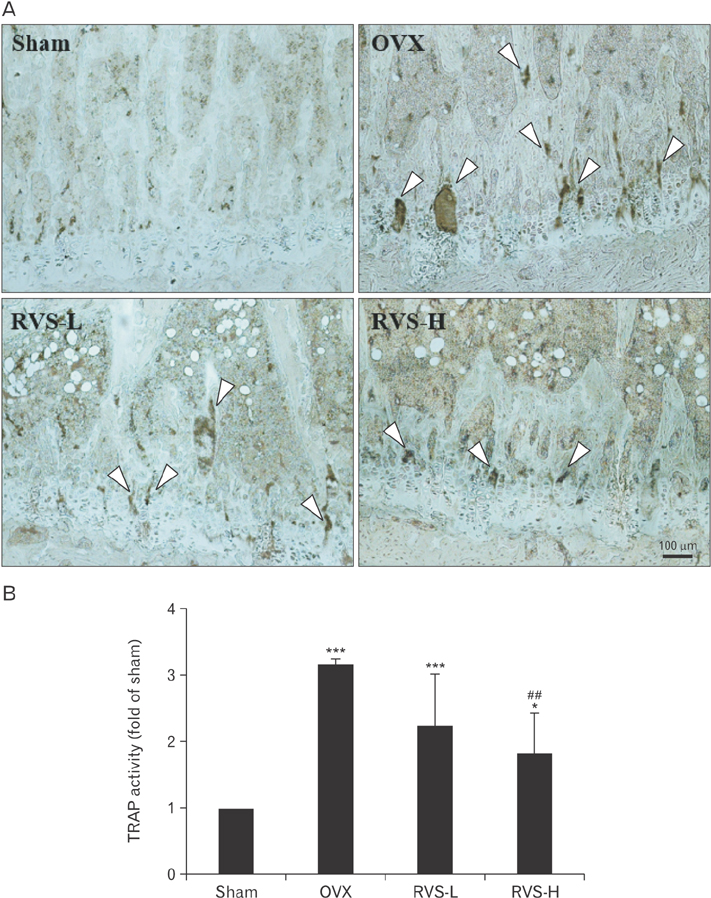
- Post-menopausal osteoporosis (PMO) is a major global human health concern. Owing to the need for therapeutic drugs without side effects, natural extracts containing various polyphenolic compounds that may exert estrogenic effects have been studied in depth. Rhus verniciflua Stokes (RVS), which has been used as a traditional herbal medicine for centuries in Korea, was recently revealed to exert estrogenic effects attributable to its bioactive ingredients sulfuretin and butein, which have strong estrogen receptor-binding affinities. In this study, the protective potential of RVS in PMO was evaluated by using an experimental animal model of PMO, which was established by ovariectomy (OVX) of female Sprague Dawley rats. The oral administration of RVS at 20 mg/kg or 100 mg/kg for 8 weeks markedly protected against OVX-induced atrophy of the uterine tube and reversed the elevation in the ratio of serum receptor activator of nuclear factor-κB ligand to osteoprotegerin, which is a marker of disease severity. In addition, RVS inhibited OVX-induced tibia bone loss, activated osteogenic activity, and suppressed osteoclastic activity in the tibial epiphyseal plate, a region of bone remodeling. Collectively, these factors indicated that the oral intake of RVS might be beneficial for the prevention of PMO.
MeSH Terms
Figure
Reference
-
1. Bernabei R, Martone AM, Ortolani E, Landi F, Marzetti E. Screening, diagnosis and treatment of osteoporosis: a brief review. Clin Cases Miner Bone Metab. 2014; 11:201–207.2. Greenwald MW, Gluck OS, Lang E, Rakov V. Oral hormone therapy with 17beta-estradiol and 17beta-estradiol in combination with norethindrone acetate in the prevention of bone loss in early postmenopausal women: dose-dependent effects. Menopause. 2005; 12:741–748.3. Imai Y, Kondoh S, Kouzmenko A, Kato S. Minireview: osteoprotective action of estrogens is mediated by osteoclastic estrogen receptor-alpha. Mol Endocrinol. 2010; 24:877–885.4. Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H, Stein GS, van Wijnen AJ, Lanyon LE, Price JS. Estrogen receptor alpha mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor beta. J Biol Chem. 2013; 288:9035–9048.5. Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012; 23:576–581.6. Na KS, Jee DH, Han K, Park YG, Kim MS, Kim EC. The ocular benefits of estrogen replacement therapy: a population-based study in postmenopausal Korean women. PLoS One. 2014; 9:e106473.7. Fernandez E, Gallus S, Bosetti C, Franceschi S, Negri E, La Vecchia C. Hormone replacement therapy and cancer risk: a systematic analysis from a network of case-control studies. Int J Cancer. 2003; 105:408–412.8. Humphries KH, Gill S. Risks and benefits of hormone replacement therapy: the evidence speaks. CMAJ. 2003; 168:1001–1010.9. Gencel VB, Benjamin MM, Bahou SN, Khalil RA. Vascular effects of phytoestrogens and alternative menopausal hormone therapy in cardiovascular disease. Mini Rev Med Chem. 2012; 12:149–174.10. Miksicek RJ. Commonly occurring plant flavonoids have estrogenic activity. Mol Pharmacol. 1993; 44:37–43.11. Sulaiman CT, Arun A, Anandan EM, Sandhya CR, Balachandran I. Isolation and identification of phytoestrogens and flavonoids in an Ayurvedic proprietary medicine using chromatographic and mass spectroscopic analysis. Asian Pac J Reprod. 2015; 4:153–156.12. Kitts DD, Lim KT. Antitumorigenic and cytotoxic properties of an ethanol extract derived from Rhus verniciflua Stokes (RVS). J Toxicol Environ Health A. 2001; 64:357–371.13. Hong DH, Han SB, Lee CW, Park SH, Jeon YJ, Kim MJ, Kwak SS, Kim HM. Cytotoxicity of urushiols isolated from sap of Korean lacquer tree (Rhus vernicifera Stokes). Arch Pharm Res. 1999; 22:638–641.14. Park JM, Lee JH, Na CS, Lee D, Lee JY, Satoh M, Lee MY. Heartwood extract of Rhus verniciflua Stokes and its active constituent fisetin attenuate vasoconstriction through calcium-dependent mechanism in rat aorta. Biosci Biotechnol Biochem. 2016; 80:493–500.15. Park SD, Lee SW, Chun JH, Cha SH. Clinical features of 31 patients with systemic contact dermatitis due to the ingestion of Rhus (lacquer). Br J Dermatol. 2000; 142:937–942.16. Shin JS, Park YM, Choi JH, Park HJ, Shin MC, Lee YS, Lee KT. Sulfuretin isolated from heartwood of Rhus verniciflua inhibits LPS-induced inducible nitric oxide synthase, cyclooxygenase-2, and pro-inflammatory cytokines expression via the down-regulation of NF-kappaB in RAW 264.7 murine macrophage cells. Int Immunopharmacol. 2010; 10:943–950.17. Lee JD, Huh JE, Jeon G, Yang HR, Woo HS, Choi DY, Park DS. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int Immunopharmacol. 2009; 9:268–276.18. Kim JH, Jung CH, Jang BH, Go HY, Park JH, Choi YK, Hong SI, Shin YC, Ko SG. Selective cytotoxic effects on human cancer cell lines of phenolic-rich ethyl-acetate fraction from Rhus verniciflua Stokes. Am J Chin Med. 2009; 37:609–620.19. Lim KT, Hu C, Kitts DD. Antioxidant activity of a Rhus verniciflua Stokes ethanol extract. Food Chem Toxicol. 2001; 39:229–237.20. Sun S, Choi YH, Na CS, Lee D, Yoo HH, Hong CY, Ahn BY, Dong MS. Estrogenic activity of a Rhus verniciflua extract and its major components. J Funct Foods. 2014; 11:250–260.21. Gil MN, Choi DR, Yu KS, Jeong JH, Bak DH, Kim DK, Lee NS, Lee JH, Jeong YG, Na CS, Na DS, Ryu KH, Han SY. Rhus verniciflua Stokes attenuates cholestatic liver cirrhosis-induced interstitial fibrosis via Smad3 down-regulation and Smad7 up-regulation. Anat Cell Biol. 2016; 49:189–198.22. Genant HK, Engelke K, Prevrhal S. Advanced CT bone imaging in osteoporosis. Rheumatology (Oxford). 2008; 47:Suppl 4. iv9–iv16.23. Du J, Feng W, Sun J, Kang C, Amizuka N, Li M. Ovariectomy upregulated the expression of peroxiredoxin 1 &5 in osteoblasts of mice. Sci Rep. 2016; 6:35995.24. Kim W, Bae S, Kim H, Kim Y, Choi J, Lim SY, Lee HJ, Lee J, Jang M, Lee KE, Chung SG, Hwang YI, Kang JS, Lee WJ. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anat Cell Biol. 2013; 46:254–261.25. Xie F, Wu CF, Lai WP, Yang XJ, Cheung PY, Yao XS, Leung PC, Wong MS. The osteoprotective effect of Herba epimedii (HEP) extract in vivo and in vitro. Evid Based Complement Alternat Med. 2005; 2:353–361.26. Gjoksi B, Ghayor C, Siegenthaler B, Ruangsawasdi N, Zenobi-Wong M, Weber FE. The epigenetically active small chemical N-methyl pyrrolidone (NMP) prevents estrogen depletion induced osteoporosis. Bone. 2015; 78:114–121.27. Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014; 5:511.28. Babaji P, Devanna R, Jagtap K, Chaurasia VR, Jerry JJ, Choudhury BK, Duhan D. The cell biology and role of resorptive cells in diseases: a review. Ann Afr Med. 2017; 16:39–45.29. van Lenten BJ, Melchior GW, Roheim PS. Lipoprotein metabolism in the ovariectomized rat. J Lipid Res. 1983; 24:1475–1484.30. Kikuchi-Hayakawa H, Onodera N, Matsubara S, Yasuda E, Chonan O, Takahashi R, Ishikawa F. Effects of soy milk and bifidobacterium fermented soy milk on lipid metabolism in aged ovariectomized rats. Biosci Biotechnol Biochem. 1998; 62:1688–1692.31. Liu D, Bachmann KA. An investigation of the relationship between estrogen, estrogen metabolites and blood cholesterol levels in ovariectomized rats. J Pharmacol Exp Ther. 1998; 286:561–568.32. Lucas EA, Juma S, Stoecker BJ, Arjmandi BH. Prune suppresses ovariectomy-induced hypercholesterolemia in rats. J Nutr Biochem. 2000; 11:255–259.33. Spady DK, Bilheimer DW, Dietschy JM. Rates of receptor-dependent and -independent low density lipoprotein uptake in the hamster. Proc Natl Acad Sci U S A. 1983; 80:3499–3503.34. Sznajderman M, Oliver MF. Spontaneous premature menopause, ischemic heart-disease, and serum-lipids. Lancet. 1963; 281:962–965.35. Rosenberg L, Hennekens CH, Rosner B, Belanger C, Rothman KJ, Speizer FE. Early menopause and the risk of myocardial infarction. Am J Obstet Gynecol. 1981; 139:47–51.36. Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998; 95:3597–3602.37. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.38. Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999; 190:1741–1754.39. Lee SE, Woo KM, Kim SY, Kim HM, Kwack K, Lee ZH, Kim HH. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 2002; 30:71–77.40. Takatsuna H, Asagiri M, Kubota T, Oka K, Osada T, Sugiyama C, Saito H, Aoki K, Ohya K, Takayanagi H, Umezawa K. Inhibition of RANKL-induced osteoclastogenesis by (-)-DHMEQ, a novel NF-kappaB inhibitor, through downregulation of NFATc1. J Bone Miner Res. 2005; 20:653–662.41. Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007; 1116:227–237.42. Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem Biophys Res Commun. 1998; 250:776–781.43. Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999; 25:255–259.44. Jung CH, Jun CY, Lee S, Park CH, Cho K, Ko SG. Rhus verniciflua Stokes extract: radical scavenging activities and protective effects on H2O2-induced cytotoxicity in macrophage RAW 264.7 cell lines. Biol Pharm Bull. 2006; 29:1603–1607.45. Park BC, Lee YS, Park HJ, Kwak MK, Yoo BK, Kim JY, Kim JA. Protective effects of fustin, a flavonoid from Rhus verniciflua Stokes, on 6-hydroxydopamine-induced neuronal cell death. Exp Mol Med. 2007; 39:316–326.46. Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013; 60:1–4.47. Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010; 31:266–300.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experimental Study on Latent Sensitivity to Rhus Trees
- The Effect of Rhus verniciflua Stokes Extracts on Photo-Aged Mouse Skin
- Rhus verniciflua Stokes extract suppresses migration and invasion in human gastric adenocarcinoma AGS cells
- Effect of Polyurushiol Paint on Indoor Air Quality and Atopic Dermatitis
- Urushiol V Suppresses Cell Proliferation and Enhances Antitumor Activity of 5-FU in Human Colon Cancer Cells by Downregulating FoxM1