Ann Dermatol.
2017 Oct;29(5):529-535. 10.5021/ad.2017.29.5.529.
Thiopurine S-Methyltransferase Polymorphisms in Korean Dermatologic Patients
- Affiliations
-
- 1Department of Dermatology, Severance Hospital, Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea. dykim@ yuhs.ac
- 2Department of Dermatology, Catholic Kwandong University International St. Mary's Hospital, Incheon, Korea.
- KMID: 2388902
- DOI: http://doi.org/10.5021/ad.2017.29.5.529
Abstract
- BACKGROUND
Thiopurine S-methyltransferase (TPMT) is an important enzyme in the metabolism of thiopurines including azathioprine (AZA), 6-mercaptopurine, and 6-thioguanine. TPMT genotyping is widely used for screening of AZA-related toxicity during routine clinical practice in Korea. However, the data of TPMT genotypes and its AZA-related toxicity have not been studied in the field of dermatology.
OBJECTIVE
The aim of this study was to evaluate the genetic basis of TPMT polymorphism in Korean dermatologic patients and subsequently to investigate the relationship between mutant TPMT and adverse responses to AZA treatment.
METHODS
This study was retrospective, single-center study. One hundred forty-nine Korean dermatologic patients who underwent TPMT screening test were included. Each patient's medical records, the result of TPMT screening test, dose and treatment period of AZA, and side effects, were reviewed. Laboratory tests were assessed at each visit in order to monitor adverse drug reactions. Leukopenia grading was used in accordance with the common terminology criteria for adverse events (CTCAE) ver. 4.03.
RESULTS
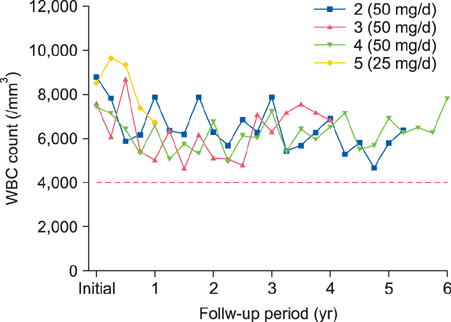
Behçet's disease was the leading disorder among the patients. The frequency of TPMT mutation was 4.0% (6/149) among the participants in this study. Four of the six patients with genetic alterations were treated with a low-dose AZA regimen, but no AZA-related adverse events were observed.
CONCLUSION
Our results suggest that 1) TPMT polymorphisms in Korean dermatologic patients are similar to those previously reported in Asian patients with the most common mutant allele being TPMT*3C and 2) AZA can be used in the patients with these polymorphisms under a careful dosing regimen.
MeSH Terms
Figure
Reference
-
1. Sandborn W, Sutherland L, Pearson D, May G, Modigliani R, Prantera C. Azathioprine or 6-mercaptopurine for inducing remission of Crohn's disease. Cochrane Database Syst Rev. 2000; (2):CD000545.2. Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006; 41:10–16.
Article3. Patel AA, Swerlick RA, McCall CO. Azathioprine in dermatology: the past, the present, and the future. J Am Acad Dermatol. 2006; 55:369–389.
Article4. Gisbert JP, Gomollón F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008; 103:1783–1800.
Article5. Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989; 46:149–154.
Article6. Yates CR, Krynetski EY, Loennechen T, Fessing MY, Tai HL, Pui CH, et al. Molecular diagnosis of thiopurine Smethyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997; 126:608–614.
Article7. Marinaki AM, Duley JA, Arenas M, Ansari A, Sumi S, Lewis CM, et al. Mutation in the ITPA gene predicts intolerance to azathioprine. Nucleosides Nucleotides Nucleic Acids. 2004; 23:1393–1397.8. Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014; 46:1017–1020.
Article9. Krynetskaia NF, Krynetski EY, Evans WE. Human RNase H-mediated RNA cleavage from DNA-RNA duplexes is inhibited by 6-deoxythioguanosine incorporation into DNA. Mol Pharmacol. 1999; 56:841–848.10. Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000; 118:705–713.
Article11. Appell ML, Berg J, Duley J, Evans WE, Kennedy MA, Lennard L, et al. Nomenclature for alleles of the thiopurine methyltransferase gene. Pharmacogenet Genomics. 2013; 23:242–248.
Article12. Salavaggione OE, Wang L, Wiepert M, Yee VC, Weinshilboum RM. Thiopurine S-methyltransferase pharmacogenetics: variant allele functional and comparative genomics. Pharmacogenet Genomics. 2005; 15:801–815.
Article13. Ishioka S, Hiyama K, Sato H, Yamanishi Y, McLeod HL, Kumagai K, et al. Thiopurine methyltransferase genotype and the toxicity of azathioprine in Japanese. Intern Med. 1999; 38:944–947.
Article14. Cao Q, Zhu Q, Shang Y, Gao M, Si J. Thiopurine methyltransferase gene polymorphisms in Chinese patients with inflammatory bowel disease. Digestion. 2009; 79:58–63.
Article15. Jun JB, Cho DY, Kang C, Bae SC. Thiopurine S-methyltransferase polymorphisms and the relationship between the mutant alleles and the adverse effects in systemic lupus erythematosus patients taking azathioprine. Clin Exp Rheumatol. 2005; 23:873–876.16. Kim MJ, Lee SY, Choe YH. Monitoring thiopurine metabolites in Korean pediatric patients with inflammatory bowel disease. Yonsei Med J. 2014; 55:1289–1296.
Article17. Kim JH, Cheon JH, Hong SS, Eun CS, Byeon JS, Hong SY, et al. Influences of thiopurine methyltransferase genotype and activity on thiopurine-induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J Clin Gastroenterol. 2010; 44:e242–e248.18. Jung YS, Cheon JH, Park JJ, Moon CM, Kim ES, Lee JH, et al. Correlation of genotypes for thiopurine methyltransferase and inosine triphosphate pyrophosphatase with long-term clinical outcomes in Korean patients with inflammatory bowel diseases during treatment with thiopurine drugs. J Hum Genet. 2010; 55:121–123.
Article19. Kham SK, Tan PL, Tay AH, Heng CK, Yeoh AE, Quah TC. Thiopurine methyltransferase polymorphisms in a multiracial Asian population and children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2002; 24:353–359.
Article20. Otterness D, Szumlanski C, Lennard L, Klemetsdal B, Aarbakke J, Park-Hah JO, et al. Human thiopurine methyltransferase pharmacogenetics: gene sequence polymorphisms. Clin Pharmacol Ther. 1997; 62:60–73.
Article21. Lee SS, Kim WY, Jang YJ, Shin JG. Duplex pyrosequencing of the TPMT*3C and TPMT*6 alleles in Korean and Vietnamese populations. Clin Chim Acta. 2008; 398:82–85.
Article22. Lowry PW, Franklin CL, Weaver AL, Szumlanski CL, Mays DC, Loftus EV, et al. Leucopenia resulting from a drug interaction between azathioprine or 6-mercaptopurine and mesalamine, sulphasalazine, or balsalazide. Gut. 2001; 49:656–664.
Article23. Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011; 89:387–391.
Article24. Black AJ, McLeod HL, Capell HA, Powrie RH, Matowe LK, Pritchard SC, et al. Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann Intern Med. 1998; 129:716–718.
Article25. Dubinsky MC, Reyes E, Ofman J, Chiou CF, Wade S, Sandborn WJ. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn's disease treated with azathioprine or 6-mercaptopurine. Am J Gastroenterol. 2005; 100:2239–2247.
Article26. Cuffari C, Dassopoulos T, Turnbough L, Thompson RE, Bayless TM. Thiopurine methyltransferase activity influences clinical response to azathioprine in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004; 2:410–417.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NUDT15 gene variants and thiopurine-induced leukopenia in patients with inflammatory bowel disease
- Thiopurine S-methyltransferase Polymorphisms and the Relationship between the Mutant Alleles and the Adverse Effects in Systemic Lupus Erythematosus Patients Taking Azathioprine
- NUDT15 Genotyping in Thiopurine Drug Therapy
- Genetic Polymorphism of Thiopurine Methyltransferase in Children with Acute Lymphoblastic Leukemia
- Association between COMT and 5-HTTLPR Polymorphisms in Korean Patients with Panic Disorder : A Replication Study