NUDT15 gene variants and thiopurine-induced leukopenia in patients with inflammatory bowel disease
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Toho University Sakura Medical Center, Sakura, Japan
- KMID: 2504582
- DOI: http://doi.org/10.5217/ir.2020.00002
Abstract
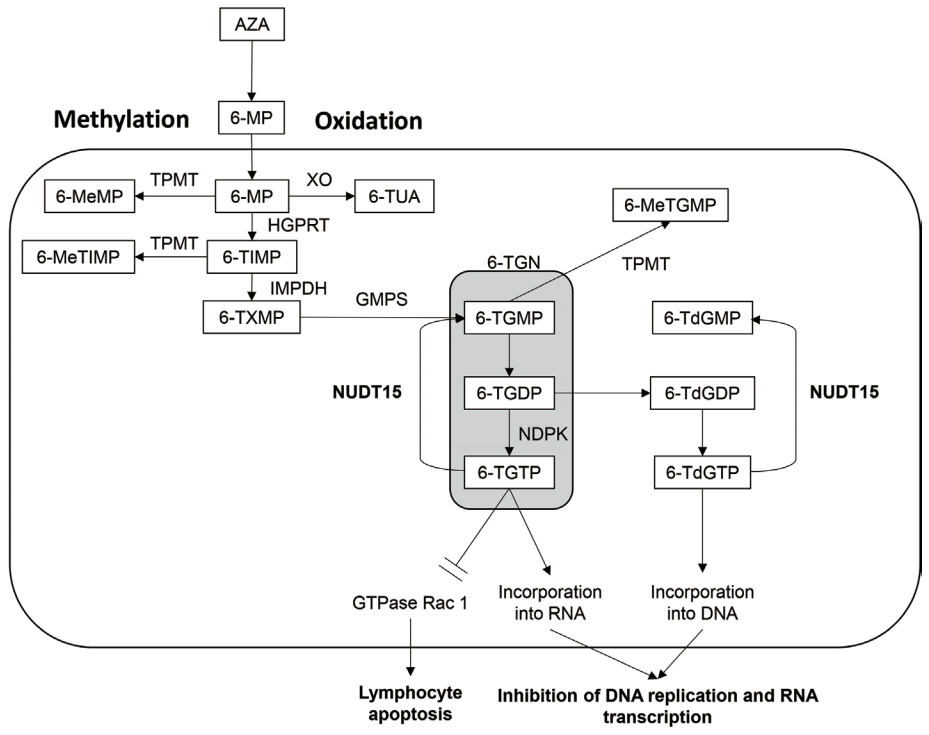
- Thiopurine has been used to maintain remission and to reduce antidrug antibody formation in monoclonal antibody therapy in patients with inflammatory bowel disease (IBD). The use of thiopurine is limited by side effects such as leukopenia. Thiopurine S-methyltransferase (TPMT) variants are associated with thiopurine-induced leukopenia in Westerners, but the frequency of the risk alleles is low in Asians. Recently, a variant in the nudix hydrolase 15 (NUDT15) gene (R139C, c.415C > T) was reported to be associated with early severe leukopenia in Asians. NUDT15 is an enzyme that converts 6-thio-(deoxy)guanosine triphosphate (6-T(d)GTP) to 6-thio-(deoxy)guanosine monophosphate (6-T(d)GMTP). The R139C variant impairs the stability of the protein and increases incorporation of 6-TGTP and 6-TdGTP into RNA and DNA, respectively, resulting in leukopenia. The frequency of C/C, C/T, and T/T are approximately 80%, 20%, and 1%, respectively in East Asians. Early leukopenia occurred in less than 3% of patients with C/C and in around 20% of those with C/T, whereas it occurred in almost all patients with T/T. Patients homozygous for this variant also develop severe hair loss. The measurement of NUDT15 R139C can increase the safety of thiopurine dramatically and is a successful example of personalized medicine in the field of IBD.
Keyword
Figure
Cited by 4 articles
-
Long-term efficacy and tolerability of dose-adjusted thiopurine treatment in maintaining remission in inflammatory bowel disease patients with
NUDT15 heterozygosity
Takato Maeda, Hirotake Sakuraba, Hiroto Hiraga, Shukuko Yoshida, Yoichi Kakuta, Hidezumi Kikuchi, Shogo Kawaguchi, Keisuke Hasui, Tetsuya Tatsuta, Daisuke Chinda, Tatsuya Mikami, Shinsaku Fukuda
Intest Res. 2022;20(1):90-100. doi: 10.5217/ir.2020.00133.Use of thiopurines in inflammatory bowel disease: an update
Arshdeep Singh, Ramit Mahajan, Saurabh Kedia, Amit Kumar Dutta, Abhinav Anand, Charles N. Bernstein, Devendra Desai, C. Ganesh Pai, Govind Makharia, Harsh Vardhan Tevethia, Joyce WY Mak, Kirandeep Kaur, Kiran Peddi, Mukesh Kumar Ranjan, Perttu Arkkila, Rakesh Kochhar, Rupa Banerjee, Saroj Kant Sinha, Siew Chien Ng, Stephen Hanauer, Suhang Verma, Usha Dutta, Vandana Midha, Varun Mehta, Vineet Ahuja, Ajit Sood
Intest Res. 2022;20(1):11-30. doi: 10.5217/ir.2020.00155.Natural history of inflammatory bowel disease: a comparison between the East and the West
Eun Mi Song, Suk-Kyun Yang
Intest Res. 2022;20(4):418-430. doi: 10.5217/ir.2021.00104.Treatment of Autoimmune Hepatitis
Ja Kyung Kim
Korean J Gastroenterol. 2023;81(2):72-85. doi: 10.4166/kjg.2023.011.
Reference
-
1. Burchenal JH, Murphy ML, Ellison RR, et al. Clinical evaluation of a new antimetabolite, 6-mercaptopurine, in the treatment of leukemia and allied diseases. Blood. 1953; 8:965–999.
Article2. Schwartz R, Stack J, Dameshek W. Effect of 6-mercaptopurine on antibody production. Proc Soc Exp Biol Med. 1958; 99:164–167.
Article3. Bowen GE, Irons GV Jr, Rhodes JB, Kirsner JB. Early experiences with azathioprine in ulcerative colitis; a note of caution. JAMA. 1966; 195:460–464.
Article4. Takatsu N, Matsui T, Murakami Y, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2009; 24:1258–1264.
Article5. Qiu Y, Mao R, Zhang SH, et al. Safety profile of thiopurines in Crohn disease: analysis of 893 patient-years follow-up in a Southern China Cohort. Medicine (Baltimore). 2015; 94:e1513.6. Kim JH, Cheon JH, Hong SS, et al. Influences of thiopurine methyltransferase genotype and activity on thiopurine-induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J Clin Gastroenterol. 2010; 44:e242–e248.7. Sood R, Ansari S, Clark T, Hamlin PJ, Ford AC. Long-term efficacy and safety of azathioprine in ulcerative colitis. J Crohns Colitis. 2015; 9:191–197.
Article8. Lewis JD, Abramson O, Pascua M, et al. Timing of myelosuppression during thiopurine therapy for inflammatory bowel disease: implications for monitoring recommendations. Clin Gastroenterol Hepatol. 2009; 7:1195–1201.
Article9. Asada A, Nishida A, Shioya M, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016; 51:22–29.
Article10. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68(Suppl 3):s1–s106.
Article11. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article12. Relling MV, Schwab M, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 2019; 105:1095–1105.
Article13. Fangbin Z, Xiang G, Minhu C, et al. Should thiopurine methyltransferase genotypes and phenotypes be measured before thiopurine therapy in patients with inflammatory bowel disease? Ther Drug Monit. 2012; 34:695–701.
Article14. Collie-Duguid ES, Pritchard SC, Powrie RH, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics. 1999; 9:37–42.
Article15. Kumagai K, Hiyama K, Ishioka S, et al. Allelotype frequency of the thiopurine methyltransferase (TPMT) gene in Japanese. Pharmacogenetics. 2001; 11:275–278.
Article16. Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014; 46:1017–1020.
Article17. Tanaka Y, Kato M, Hasegawa D, et al. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol. 2015; 171:109–115.
Article18. Moon W, Loftus EV Jr. Review article: recent advances in pharmacogenetics and pharmacokinetics for safe and effective thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2016; 43:863–883.
Article19. Lim SZ, Chua EW. Revisiting the role of thiopurines in inflammatory bowel disease through pharmacogenomics and use of novel methods for therapeutic drug monitoring. Front Pharmacol. 2018; 9:1107.
Article20. Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003; 111:1133–1145.
Article21. Carter M, Jemth AS, Hagenkort A, et al. Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2. Nat Commun. 2015; 6:7871.
Article22. Valerie NC, Hagenkort A, Page BD, et al. NUDT15 hydrolyzes 6-thio-deoxyGTP to mediate the anticancer efficacy of 6-thioguanine. Cancer Res. 2016; 76:5501–5511.
Article23. Singh M, Bhatia P, Khera S, Trehan A. Emerging role of NUDT15 polymorphisms in 6-mercaptopurine metabolism and dose related toxicity in acute lymphoblastic leukaemia. Leuk Res. 2017; 62:17–22.
Article24. Man P, Fábry M, Sieglová I, Kavan D, Novák P, Hnízda A. Thiopurine intolerance-causing mutations in NUDT15 induce temperature-dependent destabilization of the catalytic site. Biochim Biophys Acta Proteins Proteom. 2019; 1867:376–381.
Article25. Nishii R, Moriyama T, Janke LJ, et al. Preclinical evaluation of NUDT15-guided thiopurine therapy and its effects on toxicity and antileukemic efficacy. Blood. 2018; 131:2466–2474.26. Tatsumi G, Kawahara M, Imai T, et al. Thiopurine-mediated impairment of hematopoietic stem and leukemia cells in Nudt15R138C knock-in mice. Leukemia. 2020; 34:882–894.
Article27. Cai JP, Ishibashi T, Takagi Y, Hayakawa H, Sekiguchi M. Mouse MTH2 protein which prevents mutations caused by 8-oxoguanine nucleotides. Biochem Biophys Res Commun. 2003; 305:1073–1077.
Article28. Chang JY, Park SJ, Jung ES, et al. Genotype-based treatment with thiopurine reduces incidence of myelosuppression in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. [published online ahead of print August 22, 2019]. https://doi.org/10.1016/j.cgh.2019.08.034.
Article29. Lee JH, Kim TJ, Kim ER, et al. Measurements of 6-thioguanine nucleotide levels with TPMT and NUDT15 genotyping in patients with Crohn’s disease. PLoS One. 2017; 12:e0188925.
Article30. Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016; 16:280–285.
Article31. Sato T, Takagawa T, Kakuta Y, et al. NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases. Intest Res. 2017; 15:328–337.
Article32. Chao K, Wang X, Cao Q, et al. Combined detection of NUDT15 variants could highly predict thiopurine-induced leukopenia in Chinese patients with inflammatory bowel disease: a multicenter analysis. Inflamm Bowel Dis. 2017; 23:1592–1599.
Article33. Sutiman N, Chen S, Ling KL, et al. Predictive role of NUDT15 variants on thiopurine-induced myelotoxicity in Asian inflammatory bowel disease patients. Pharmacogenomics. 2018; 19:31–43.
Article34. Shah SA, Paradkar M, Desai D, Ashavaid TF. Nucleoside diphosphate-linked moiety X-type motif 15 C415T variant as a predictor for thiopurine-induced toxicity in Indian patients. J Gastroenterol Hepatol. 2017; 32:620–624.
Article35. Suarez-Kurtz G, Brisson GD, Hutz MH, Petzl-Erler ML, Salzano FM. NUDT15 polymorphism in native American populations of Brazil. Clin Pharmacol Ther. 2019; 105:1321–1322.36. Jarrar YB, Ghishan M. The nudix hydrolase 15 (NUDT15) gene variants among Jordanian Arab population. Asian Pac J Cancer Prev. 2019; 20:801–808.
Article37. Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016; 48:367–373.
Article38. Walker GJ, Harrison JW, Heap GA, et al. Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA. 2019; 321:773–785.39. Kakuta Y, Kinouchi Y, Shimosegawa T. Pharmacogenetics of thiopurines for inflammatory bowel disease in East Asia: prospects for clinical application of NUDT15 genotyping. J Gastroenterol. 2018; 53:172–180.
Article40. Kakuta Y, Kawai Y, Okamoto D, et al. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. 2018; 53:1065–1078.
Article41. Akiyama S, Matsuoka K, Fukuda K, et al. Long-term effect of NUDT15 R139C on hematologic indices in inflammatory bowel disease patients treated with thiopurine. J Gastroenterol Hepatol. 2019; 34:1751–1757.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases
- Prevention of thiopurine-induced early leukopenia in a Korean pediatric patient with Crohn’s disease who turned out to possess homozygous mutations in NUDT15 R139C
- NUDT15 Genotyping in Thiopurine Drug Therapy
- Pharmacogenetics-based personalized treatment in patients with inflammatory bowel disease A review
- Long-term efficacy and tolerability of dose-adjusted thiopurine treatment in maintaining remission in inflammatory bowel disease patients with NUDT15 heterozygosity