Yeungnam Univ J Med.
2020 Oct;37(4):332-336. 10.12701/yujm.2020.00178.
Prevention of thiopurine-induced early leukopenia in a Korean pediatric patient with Crohn’s disease who turned out to possess homozygous mutations in NUDT15 R139C
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Korea
- 2Crohn's and Colitis Association in Daegu-Gyeongbuk (CCAiD), Daegu, Korea
- KMID: 2508180
- DOI: http://doi.org/10.12701/yujm.2020.00178
Abstract
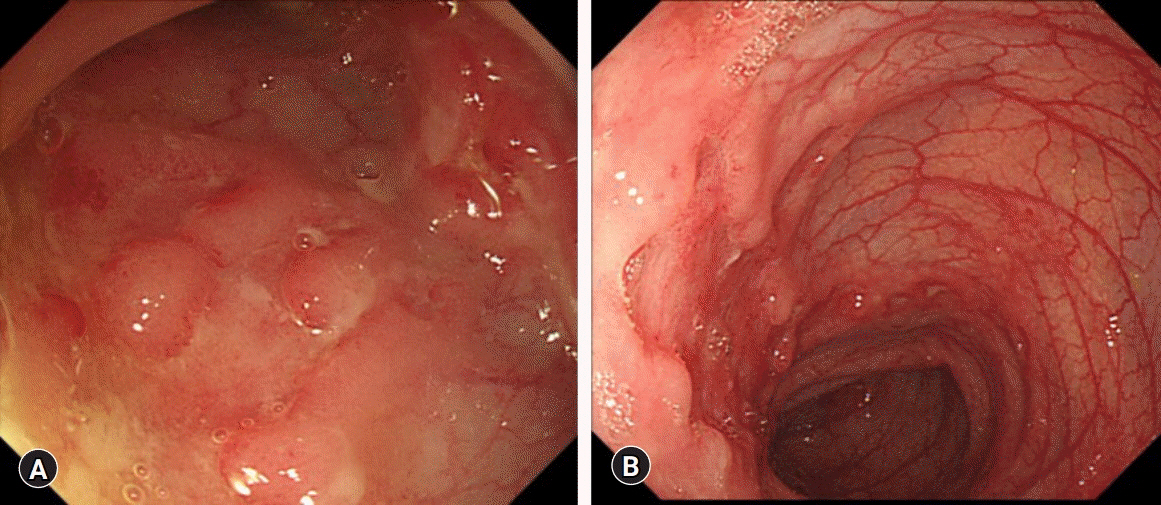
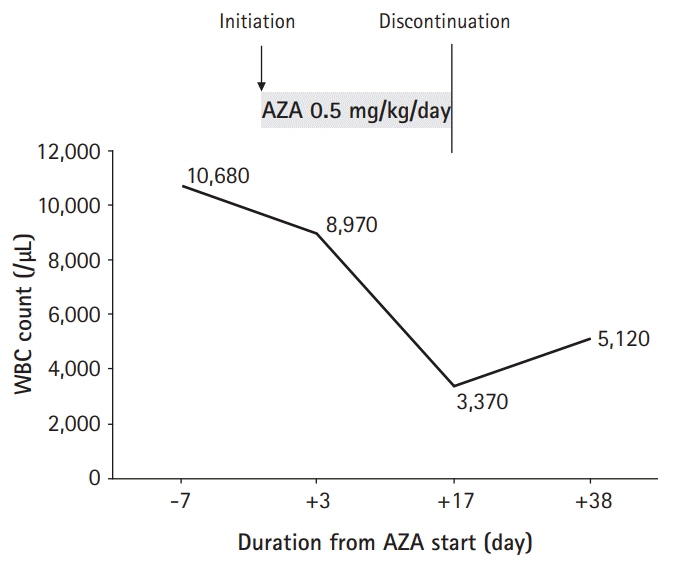
- Homozygous mutations in NUDT15 R139C are known as the major factor associated with thiopurine-induced early leukopenia, particularly in Asian patients. Therefore, NUDT15 genotyping is currently recommended before thiopurine treatment to identify patients who are NUDT15 poor metabolizers and consider the use of an alternative immunomodulatory therapy. We report a case of a 12-year-old Korean girl with Crohn’s disease (CD), in whom thiopurine-induced leukopenia was prevented by initiation of azathioprine (AZA) therapy at a low dose (0.5 mg/kg/day) and early detection of significant hair loss and white blood cell (WBC) count decrease at 17 days from the start of AZA treatment. The WBC count dropped from 8,970/μL to 3,370/μL in 2 weeks, and AZA treatment was stopped because of concerns of potential leukopenia in the near future. Her WBC count recovered to 5,120/μL after 3 weeks. Gene analysis later revealed that she had a homozygous mutation in NUDT15 R139C, resulting in a poor metabolizing activity of NUDT15. In situations when NUDT15 genotyping is unavailable, initiation of AZA therapy at 0.5 mg/kg/day with close observation of hair loss and WBC counts within 2 weeks may be an alternative way to prevent thiopurine-induced early leukopenia in Asian children with CD.
Keyword
Figure
Reference
-
References
1. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 2015; 169:1053–60.
Article2. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011; 17:423–39.
Article3. Hong SJ, Cho SM, Choe BH, Jang HJ, Choi KH, Kang B, et al. Characteristics and incidence trends for pediatric inflammatory bowel disease in Daegu-Kyungpook Province in Korea: a multi-center study. J Korean Med Sci. 2018; 33:e132.
Article4. Kang B, Kim JE, Jung JH, Choe JY, Kim MJ, Choe YH, et al. Korean children and adolescents with Crohn's disease are more likely to present with perianal fistulizing disease at diagnosis compared to their European counterparts. Pediatr Gastroenterol Hepatol Nutr. 2020; 23:49–62.
Article5. Kang B, Choi SY, Kim HS, Kim K, Lee YM, Choe YH. Mucosal healing in paediatric patients with moderate-to-severe luminal Crohn's disease under combined immunosuppression: escalation versus early treatment. J Crohns Colitis. 2016; 10:1279–86.
Article6. Kang B, Choe YH. Early biologic treatment in pediatric Crohn's disease: catching the therapeutic window of opportunity in early disease by treat-to-target. Pediatr Gastroenterol Hepatol Nutr. 2018; 21:1–11.
Article7. Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014; 8:1179–207.
Article8. Kim JH, Cheon JH, Kim WH. The frequency and the course of the adverse effects of azathioprine/6-mercaptopurine treatment in patients with inflammatory bowel disease. Korean J Gastroenterol. 2008; 51:291–7.9. Chun JY, Kang B, Lee YM, Lee SY, Kim MJ, Choe YH. Adverse events associated with azathioprine treatment in korean pediatric inflammatory bowel disease patients. Pediatr Gastroenterol Hepatol Nutr. 2013; 16:171–7.
Article10. Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014; 46:1017–20.
Article11. Lee YJ, Hwang EH, Park JH, Shin JH, Kang B, Kim SY. NUDT15 variant is the most common variant associated with thiopurine-induced early leukopenia and alopecia in Korean pediatric patients with Crohn's disease. Eur J Gastroenterol Hepatol. 2016; 28:475–8.
Article12. Kim HY, Lee SH, Lee MN, Kim JW, Kim YH, Kim MJ, et al. Complete sequence-based screening of TPMT variants in the Korean population. Pharmacogenet Genomics. 2015; 25:143–6.
Article13. Lee KM, Kim YS, Seo GS, Kim TO, Yang SK; IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of thiopurines in inflammatory bowel disease: a consensus statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 2015; 13:193–207.
Article14. Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016; 51:22–9.
Article15. Zhu X, Wang XD, Chao K, Zhi M, Zheng H, Ruan HL, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn's disease. Aliment Pharmacol Ther. 2016; 44:967–75.
Article16. Collie-Duguid ES, Pritchard SC, Powrie RH, Sludden J, Collier DA, Li T, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics. 1999; 9:37–42.
Article17. Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015; 33:1235–42.18. Kim HT, Choi R, Won HH, Choe YH, Kang B, Lee K, et al. NUDT15 genotype distributions in the Korean population. Pharmacogenet Genomics. 2017; 27:197–200.
Article19. Relling MV, Schwab M, Whirl-Carrillo M, Suarez-Kurtz G, Pui CH, Stein CM, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 2019; 105:1095–105.20. Kakuta Y, Naito T, Onodera M, Kuroha M, Kimura T, Shiga H, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016; 16:280–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NUDT15 gene variants and thiopurine-induced leukopenia in patients with inflammatory bowel disease
- NUDT15 Genotyping in Thiopurine Drug Therapy
- Pharmacogenetics-based personalized treatment in patients with inflammatory bowel disease A review
- NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases
- Long-term efficacy and tolerability of dose-adjusted thiopurine treatment in maintaining remission in inflammatory bowel disease patients with NUDT15 heterozygosity