Intest Res.
2022 Jan;20(1):90-100. 10.5217/ir.2020.00133.
Long-term efficacy and tolerability of dose-adjusted thiopurine treatment in maintaining remission in inflammatory bowel disease patients with NUDT15 heterozygosity
- Affiliations
-
- 1Department of Gastroenterology and Hematology, Hirosaki University Graduate School of Medicine, Hirosaki, Japan
- 2Shibata Irika Co. Ltd, Hirosaki, Japan
- 3Division of Gastroenterology, Tohoku University Graduate School of Medicine, Sendai, Japan
- 4Division of Endoscopy, Hirosaki University Hospital, Hirosaki, Japan
- 5Department of Vascular Biology, Hirosaki University Graduate School of Medicine, Hirosaki, Japan
- 6Department of Community Medicine, Hirosaki University Graduate School of Medicine, Hirosaki, Japan
- KMID: 2525076
- DOI: http://doi.org/10.5217/ir.2020.00133
Abstract
- Background/Aims
Thiopurines are key drugs for inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD). Recently, NUDT15 polymorphism (R139C, c.415C > T) has been shown to be associated with thiopurineinduced adverse events in Asian populations. In patients with the C/T genotype, low-dose thiopurine treatment is recommended, but its long-term efficacy and tolerability remain unclear. This study aimed to uncover the long-term efficacy and appropriate dosage of thiopurine for IBD patients with the C/T genotype.
Methods
A total of 210 patients with IBD (103 UC and 107 CD) determined to have NUDT15 R139C variants were enrolled. Clinical data were retrospectively reviewed from medical records.
Results
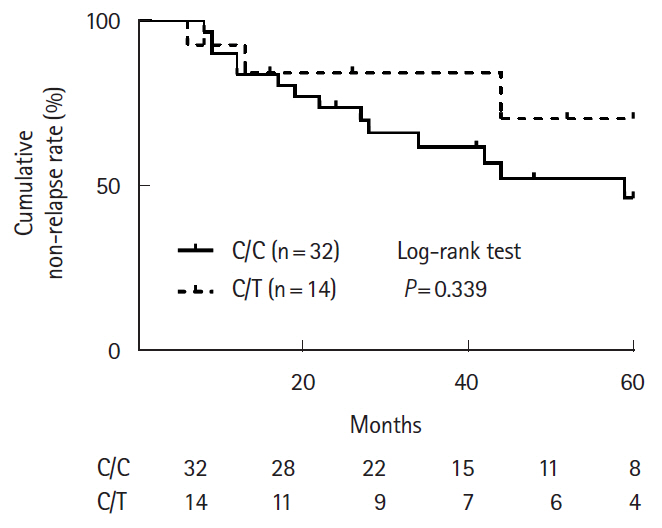
Of 46 patients (21.9%) with the C/T genotype, 30 patients (65.2%) were treated with thiopurines. Three of whom (10.0%) discontinued thiopurine treatment due to adverse events and 27 of whom continued. The median maintenance dosage of 6-mercaptopurine was 0.25 mg/kg/day (range, 0.19–0.36 mg/kg/day), and 6-thioguanine nucleotides level was 230 (104–298) pmol/8 × 108 red blood cells. Cumulative thiopurine continuation rates for 120 months for patients with the C/C and C/T genotypes were not significantly different (P= 0.895). Cumulative non-relapse rates in the patients with UC treated with thiopurine monotherapy and surgery-free rates in CD patients treated with combination therapy (thiopurines and anti-tumor necrosis factor-α agents) for maintenance remission were not significantly different at 60 months (C/C vs. C/T, P= 0.339 and P= 0.422, respectively).
Conclusions
Low-dose thiopurine treatment is an effective and acceptable treatment for patients with C/T genotype.
Figure
Reference
-
1. Gisbert JP, Linares PM, McNicholl AG, Maté J, Gomollón F. Meta-analysis: the efficacy of azathioprine and mercaptopurine in ulcerative colitis. Aliment Pharmacol Ther. 2009; 30:126–137.
Article2. Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015; (10):CD000067. Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2015;(10):CD000067.
Article3. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010; 362:1383–1395.
Article4. Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014; 146:392–400.
Article5. Qiu Y, Mao R, Chen BL, et al. Effects of combination therapy with immunomodulators on trough levels and antibodies against tumor necrosis factor antagonists in patients with inflammatory bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2017; 15:1359–1372.6. Colombel JF, Adedokun OJ, Gasink C, et al. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy: a post hoc analysis. Clin Gastroenterol Hepatol. 2019; 17:1525–1532.
Article7. Chaparro M, Ordás I, Cabré E, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013; 19:1404–1410.8. Hanauer SB, Sandborn WJ, Lichtenstein GR. Evolving considerations for thiopurine therapy for inflammatory bowel diseases: a clinical practice update: commentary. Gastroenterology. 2019; 156:36–42.
Article9. Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014; 46:1017–1020.
Article10. Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016; 16:280–285.
Article11. Kakuta Y, Kawai Y, Okamoto D, et al. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. 2018; 53:1065–1078.
Article12. Kakuta Y, Kinouchi Y, Shimosegawa T. Pharmacogenetics of thiopurines for inflammatory bowel disease in East Asia: prospects for clinical application of NUDT15 genotyping. J Gastroenterol. 2018; 53:172–180.
Article13. Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016; 48:367–373.
Article14. Matsuoka K. NUDT15 gene variants and thiopurine-induced leukopenia in patients with inflammatory bowel disease. Intest Res. 2020; 18:275–281.
Article15. Asada A, Nishida A, Shioya M, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016; 51:22–29.
Article16. Ooi CJ, Hilmi I, Banerjee R, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn’s disease in Asia. Intest Res. 2019; 17:285–310.
Article17. Akiyama S, Matsuoka K, Fukuda K, et al. Long-term effect of NUDT15 R139C on hematologic indices in inflammatory bowel disease patients treated with thiopurine. J Gastroenterol Hepatol. 2019; 34:1751–1757.
Article18. Rispo A, Testa A, De Palma GD, et al. Different profile of efficacy of thiopurines in ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2015; 21:2570–2575.
Article19. Bradford K, Shih DQ. Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease. World J Gastroenterol. 2011; 17:4166–4173.
Article20. Moreau AC, Paul S, Del Tedesco E, et al. Association between 6-thioguanine nucleotides levels and clinical remission in inflammatory disease: a meta-analysis. Inflamm Bowel Dis. 2014; 20:464–471.
Article21. Yarur AJ, Kubiliun MJ, Czul F, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015; 13:1118–1124.
Article22. Thomas CW Jr, Lowry PW, Franklin CL, et al. Erythrocyte mean corpuscular volume as a surrogate marker for 6-thioguanine nucleotide concentration monitoring in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Inflamm Bowel Dis. 2003; 9:237–245.
Article23. Moriyama T, Nishii R, Lin TN, et al. The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2017; 27:236–239.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NUDT15 gene variants and thiopurine-induced leukopenia in patients with inflammatory bowel disease
- NUDT15 Genotyping in Thiopurine Drug Therapy
- Prevention of thiopurine-induced early leukopenia in a Korean pediatric patient with Crohn’s disease who turned out to possess homozygous mutations in NUDT15 R139C
- NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases
- Pharmacogenetics-based personalized treatment in patients with inflammatory bowel disease A review