Korean J Physiol Pharmacol.
2017 Sep;21(5):495-507. 10.4196/kjpp.2017.21.5.495.
The activation of α₂-adrenergic receptor in the spinal cord lowers sepsis-induced mortality
- Affiliations
-
- 1Department of Pharmacology, Institute of Natural Medicine, College of Medicine Hallym University, Chuncheon 24252, Korea. hwsuh@hallym.ac.kr
- KMID: 2388695
- DOI: http://doi.org/10.4196/kjpp.2017.21.5.495
Abstract
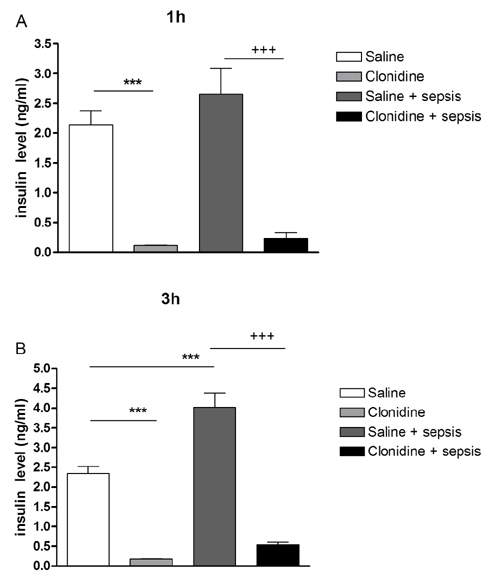
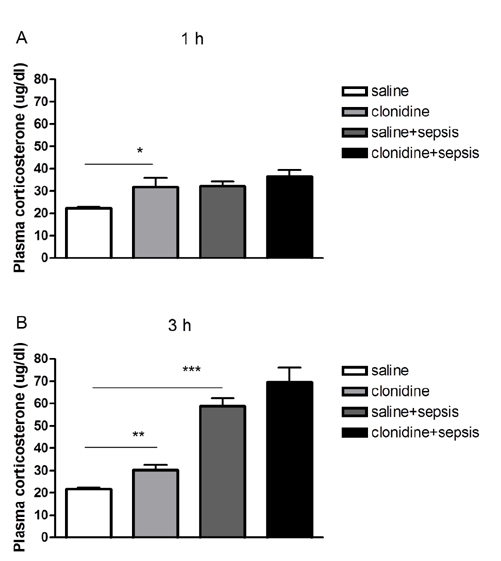
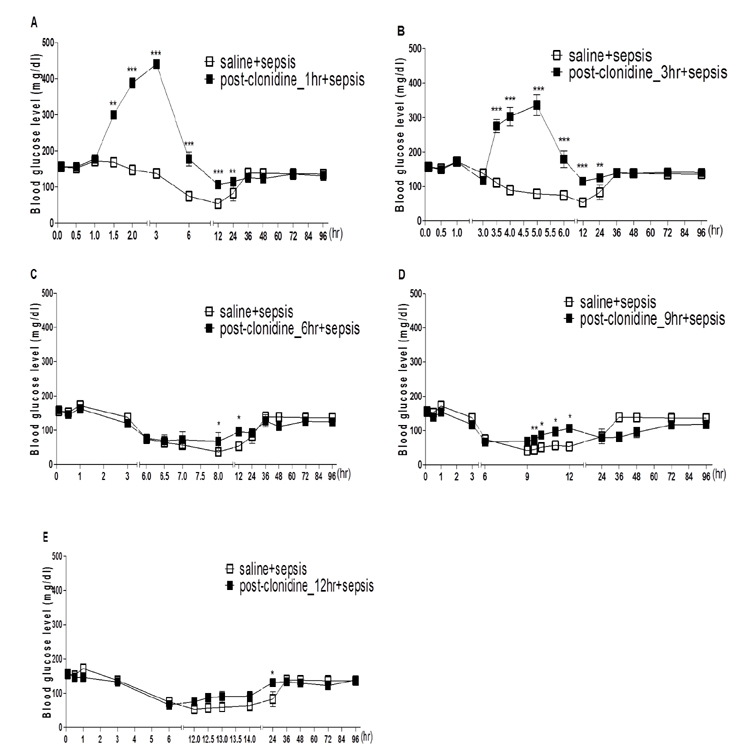
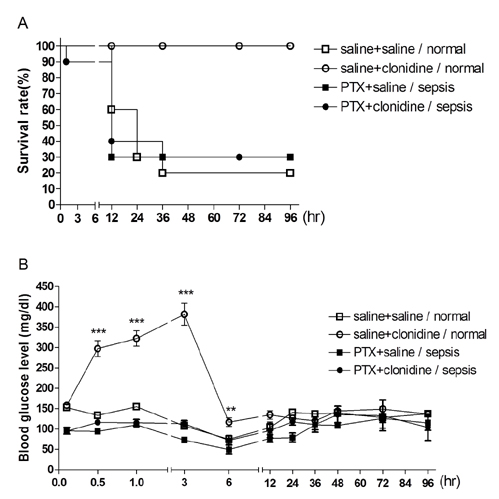
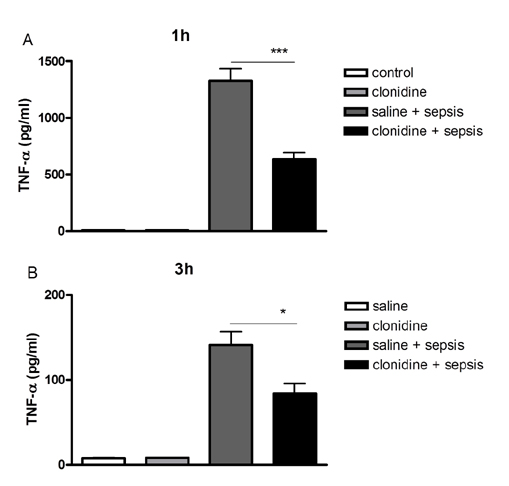
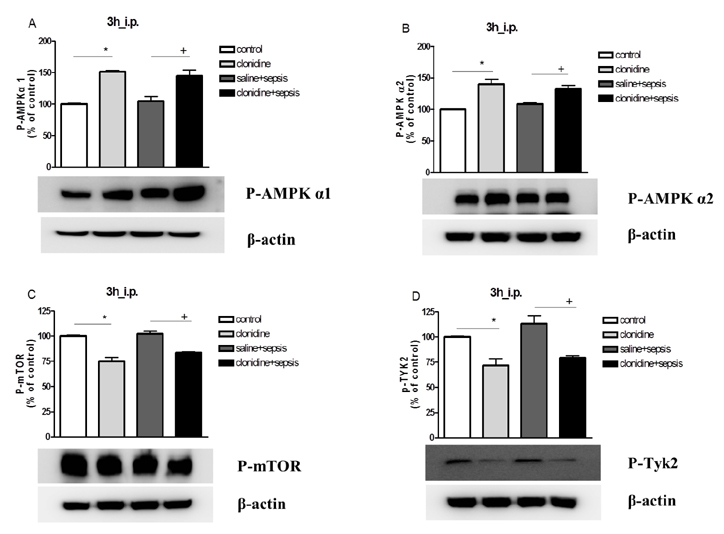
- The effect of clonidine administered intrathecally (i.t.) on the mortality and the blood glucose level induced by sepsis was examined in mice. To produce sepsis, the mixture of D-galactosamine (GaLN; 0.6 g/10 ml)/lipopolysaccharide (LPS; 27 µg/27 µl) was treated intraperitoneally (i.p.). The i.t. pretreatment with clonidine (5 µg/5 µl) increased the blood glucose level and attenuated mortality induced by sepsis in a dose-dependent manner. The i.t. post-treatment with clonidine up to 3 h caused an elevation of the blood glucose level and protected sepsis-induced mortality, whereas clonidine post-treated at 6, 9, or 12 h did not affect. The pre-treatment with oral D-glucose for 30 min prior to i.t. post-treatment (6 h) with clonidine did not rescue sepsis-induced mortality. In addition, i.t. pretreatment with pertussis toxin (PTX) reduced clonidine-induced protection against mortality and clonidine-induced hyperglycemia, suggesting that protective effect against sepsis-induced mortality seems to be mediated via activating PTX-sensitive G-proteins in the spinal cord. Moreover, pretreatment with clonidine attenuated the plasma tumor necrosis factor α (TNF-α) induced by sepsis. Clonidine administered i.t. or i.p. increased p-AMPKα1 and p-AMPKα2, but decreased p-Tyk2 and p-mTOR levels in both control and sepsis groups, suggesting that the up-regulations of p-AMPKα1 and p-AMPKα2, or down-regulations of p-mTOR and p-Tyk2 may play critical roles for the protective effect of clonidine against sepsis-induced mortality.
Keyword
MeSH Terms
Figure
Reference
-
1. Levi M, Schouten M, van der Poll T. Sepsis, coagulation, and antithrombin: old lessons and new insights. Semin Thromb Hemost. 2008; 34:742–746.2. van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, Tracey KJ, van der Poll T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005; 191:2138–2148.3. Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008; 83:536–545.4. Dünser MW, Westphal M. Arginine vasopressin in vasodilatory shock: effects on metabolism and beyond. Curr Opin Anaesthesiol. 2008; 21:122–127.5. Lang CH. Sepsis-induced insulin resistance in rats is mediated by a beta-adrenergic mechanism. Am J Physiol. 1992; 263:E703–E711.6. Maitra SR, Wojnar MM, Lang CH. Alterations in tissue glucose uptake during the hyperglycemic and hypoglycemic phases of sepsis. Shock. 2000; 13:379–385.7. Lang CH, Bagby GJ, Spitzer JJ. Glucose kinetics and body temperature after lethal and nonlethal doses of endotoxin. Am J Physiol. 1985; 248:R471–R478.8. Cherrington AD, Fuchs H, Stevenson RW, Williams PE, Alberti KG, Steiner KE. Effect of epinephrine on glycogenolysis and gluconeogenesis in conscious overnight-fasted dogs. Am J Physiol. 1984; 247:E137–E144.9. Tiruvoipati R, Chiezey B, Lewis D, Ong K, Villanueva E, Haji K, Botha J. Stress hyperglycemia may not be harmful in critically ill patients with sepsis. J Crit Care. 2012; 27:153–158.10. Pulzi Júnior SA, Assunção MS, Mazza BF, Fernandes Hda S, Jackiu M, Freitas FG, Machado FR. Accuracy of different methods for blood glucose measurement in critically ill patients. Sao Paulo Med J. 2009; 127:259–265.11. Szrama J, Smuszkiewicz P, Trojanowska I. Glycemic profile and effectiveness and safety of insulin therapy in septic patients: is the blood glucose level sufficient? Pol Arch Med Wewn. 2009; 119:621–627.12. Jensen VF, Bøgh IB, Lykkesfeldt J. Effect of insulin-induced hypoglycaemia on the central nervous system: evidence from experimental studies. J Neuroendocrinol. 2014; 26:123–150.13. Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007; 117:910–918.14. Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest. 2007; 117:868–870.15. Ogetii GN, Akech S, Jemutai J, Boga M, Kivaya E, Fegan G, Maitland K. Hypoglycaemia in severe malaria, clinical associations and relationship to quinine dosage. BMC Infect Dis. 2010; 10:334.16. Hofer S, Steppan J, Wagner T, Funke B, Lichtenstern C, Martin E, Graf BM, Bierhaus A, Weigand MA. Central sympatholytics prolong survival in experimental sepsis. Crit Care. 2009; 13:R11.17. Asano T, Dohi S, Ohta S, Shimonaka H, Iida H. Antinociception by epidural and systemic alpha(2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesth Analg. 2000; 90:400–407.18. Knaus AE, Muthig V, Schickinger S, Moura E, Beetz N, Gilsbach R, Hein L. Alpha2-adrenoceptor subtypes-unexpected functions for receptors and ligands derived from gene-targeted mouse models. Neurochem Int. 2007; 51:277–281.19. Sanders RD, Maze M. Adrenergic and cholinergic compounds. Handb Exp Pharmacol. 2007; (177):251–264.20. Lähdesmäki J, Sallinen J, MacDonald E, Sirviö J, Scheinin M. Alpha2-adrenergic drug effects on brain monoamines, locomotion, and body temperature are largely abolished in mice lacking the alpha2A-adrenoceptor subtype. Neuropharmacology. 2003; 44:882–892.21. Maze M, Scarfini C, Cavaliere F. New agents for sedation in the intensive care unit. Crit Care Clin. 2001; 17:881–897.22. Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. Substitution of a mutant alpha2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci U S A. 1997; 94:9950–9955.23. MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science. 1996; 273:801–803.24. Sim YB, Park SH, Kim SS, Lim SM, Jung JS, Suh HW. Activation of spinal α2 adrenergic receptors induces hyperglycemia in mouse though activating sympathetic outflow. Eur J Pharmacol. 2014; 741:316–322.25. Billiau A, Vandekerckhove F. Cytokines and their interactions with other inflammatory mediators in the pathogenesis of sepsis and septic shock. Eur J Clin Invest. 1991; 21:559–573.26. Zanetti G, Heumann D, Gérain J, Kohler J, Abbet P, Barras C, Lucas R, Glauser MP, Baumgartner JD. Cytokine production after intravenous or peritoneal gram-negative bacterial challenge in mice. Comparative protective efficacy of antibodies to tumor necrosis factor-alpha and to lipopolysaccharide. J Immunol. 1992; 148:1890–1897.27. Tsoyi K, Jang HJ, Nizamutdinova IT, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br J Pharmacol. 2011; 162:1498–1508.28. Escobar DA, Botero-Quintero AM, Kautza BC, Luciano J, Loughran P, Darwiche S, Rosengart MR, Zuckerbraun BS, Gomez H. Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J Surg Res. 2015; 194:262–272.29. Weichhart T, Säemann MD. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann Rheum Dis. 2008; 67:Suppl 3. iii70–iii74.30. Schaeffer V, Arbabi S, Garcia IA, Knoll ML, Cuschieri J, Bulger EM, Maier RV. Role of the mTOR pathway in LPS-activated monocytes: influence of hypertonic saline. J Surg Res. 2011; 171:769–776.31. Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007; 293:E453–E459.32. Karaghiosoff M, Steinborn R, Kovarik P, Kriegshäuser G, Baccarini M, Donabauer B, Reichart U, Kolbe T, Bogdan C, Leanderson T, Levy D, Decker T, Müller M. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol. 2003; 4:471–477.33. Bosmann M, Strobl B, Kichler N, Rigler D, Grailer JJ, Pache F, Murray PJ, Müller M, Ward PA. Tyrosine kinase 2 promotes sepsis-associated lethality by facilitating production of interleukin-27. J Leukoc Biol. 2014; 96:123–131.34. Izquierdo LA, Barros DM, Ardenghi PG, Pereira P, Rodrigues C, Choi H, Medina JH, Izquierdo I. Different hippocampal molecular requirements for short- and long-term retrieval of one-trial avoidance learning. Behav Brain Res. 2000; 111:93–98.35. Ploug T, Han X, Petersen LN, Galbo H. Effect of in vivo injection of cholera and pertussis toxin on glucose transport in rat skeletal muscle. Am J Physiol. 1997; 272:E7–E17.36. Ciaraldi TP, Maisel A. Role of guanine nucleotide regulatory proteins in insulin stimulation of glucose transport in rat adipocytes. Influence of bacterial toxins. Biochem J. 1989; 264:389–396.37. Kim CH, Park SH, Sim YB, Sharma N, Kim SS, Lim SM, Jung JS, Suh HW. Effect of pertussis and cholera toxins administered supraspinally on CA3 hippocampal neuronal cell death and the blood glucose level induced by kainic acid in mice. Neurosci Res. 2014; 89:31–36.38. Sim YB, Park SH, Kim SS, Lim SM, Jung JS, Lee JK, Suh HW. Pertussis toxin administered spinally induces a hypoglycemic effect on normal and diabetic mice. Pharmacology. 2014; 94:29–40.39. Suh HW, Sim YB, Choi YS, Song DK, Kim YH. Multiplicative interaction between intrathecally and intracerebroventricularly administered morphine for antinociception in the mouse: effects of spinally and supraspinally injected 3-isobutyl-1-methylxanthine, cholera toxin, and pertussis toxin. Gen Pharmacol. Gen Pharmacol. 1995; 26:1597–1602.40. Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981; 217:212–215.41. Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980; 67:313–316.42. Kang YJ, Sim YB, Park SH, Sharma N, Suh HW. Involvement of α2-adrenergic receptor in the regulation of the blood glucose level induced by immobilization stress. Arch Pharm Res. 2015; 38:921–929.43. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999; 96:857–868.44. Geloen A, Chapelier K, Cividjian A, Dantony E, Rabilloud M, May CN, Quintin L. Clonidine and dexmedetomidine increase the pressor response to norepinephrine in experimental sepsis: a pilot study. Crit Care Med. 2013; 41:e431–e438.45. Smythe GA, Edwards SR. A role for central postsynaptic alpha 2-adrenoceptors in glucoregulation. Brain Res. 1991; 562:225–229.46. Stump CS, Hamilton MT, Sowers JR. Effect of antihypertensive agents on the development of type 2 diabetes mellitus. Mayo Clin Proc. 2006; 81:796–806.47. Polito A, Brouland JP, Porcher R, Sonneville R, Siami S, Stevens RD, Guidoux C, Maxime V, de la Grandmaison GL, Chrétien FC, Gray F, Annane D, Sharshar T. Hyperglycaemia and apoptosis of microglial cells in human septic shock. Crit Care. 2011; 15:R131.48. Hirasawa H, Oda S, Nakamura M. Blood glucose control in patients with severe sepsis and septic shock. World J Gastroenterol. 2009; 15:4132–4136.49. Chang L, Zhao J, Yang J, Zhang Z, Du J, Tang C. Therapeutic effects of ghrelin on endotoxic shock in rats. Eur J Pharmacol. 2003; 473:171–176.50. Chang L, Du JB, Gao LR, Pang YZ, Tang CS. Effect of ghrelin on septic shock in rats. Acta Pharmacol Sin. 2003; 24:45–49.51. Kim SS, Sim YB, Park SH, Lee JR, Sharma N, Suh HW. Effect of D-glucose feeding on mortality induced by sepsis. Korean J Physiol Pharmacol. 2016; 20:83–89.52. García Hermida O, Fontela T, Ghiglione M, Uttenthal LO. Effect of pertussis pretreatment on plasma glucose and insulin responses to lithium in rats. Br J Pharmacol. 1991; 103:1309–1312.53. Toyota T, Kai Y, Kakizaki M, Sakai A, Goto Y, Yajima M, Ui M. Effects of islet-activating protein (IAP) on blood glucose and plasma insulin in healthy volunteers (phase 1 studies). Tohoku J Exp Med. 1980; 130:105–116.54. Komatsu M, McDermott AM, Gillison SL, Sharp GW. Time course of action of pertussis toxin to block the inhibition of stimulated insulin release by norepinephrine. Endocrinology. 1995; 136:1857–1863.55. Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in c and survival during sepsis in mice. Am J Physiol. 1998; 275:R269–R277.56. Lage R, Diéguez C, Vidal-Puig A, López M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008; 14:539–549.57. Vaez H, Rameshrad M, Najafi M, Barar J, Barzegari A, Garjani A. Cardioprotective effect of metformin in lipopolysaccharide-induced sepsis via suppression of toll-like receptor 4 (TLR4) in heart. Eur J Pharmacol. 2016; 772:115–123.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of D-glucose feeding on mortality induced by sepsis
- The role of spinal adrenergic receptors on the antinociception of ginsenosides in a rat postoperative pain model
- Cardiac Arrhythmias Accompanying Experimental Spinal Cord Trauma in Cats
- The Role of Adrenergic and Cholinergic Receptors on the Antinociception of Intrathecal Zaprinast in the Formalin Test of Rats
- Effect of Alpha-1-Adrenergic Agonist, Midodrine for the Management of Long-Standing Neurogenic Shock in Patient with Cervical Spinal Cord Injury: A Case Report