Korean J Neurotrauma.
2015 Oct;11(2):147-150. 10.13004/kjnt.2015.11.2.147.
Effect of Alpha-1-Adrenergic Agonist, Midodrine for the Management of Long-Standing Neurogenic Shock in Patient with Cervical Spinal Cord Injury: A Case Report
- Affiliations
-
- 1Department of Neurosurgery, National Medical Center, Seoul, Korea. chsjwa@hanmail.net
- KMID: 2378275
- DOI: http://doi.org/10.13004/kjnt.2015.11.2.147
Abstract
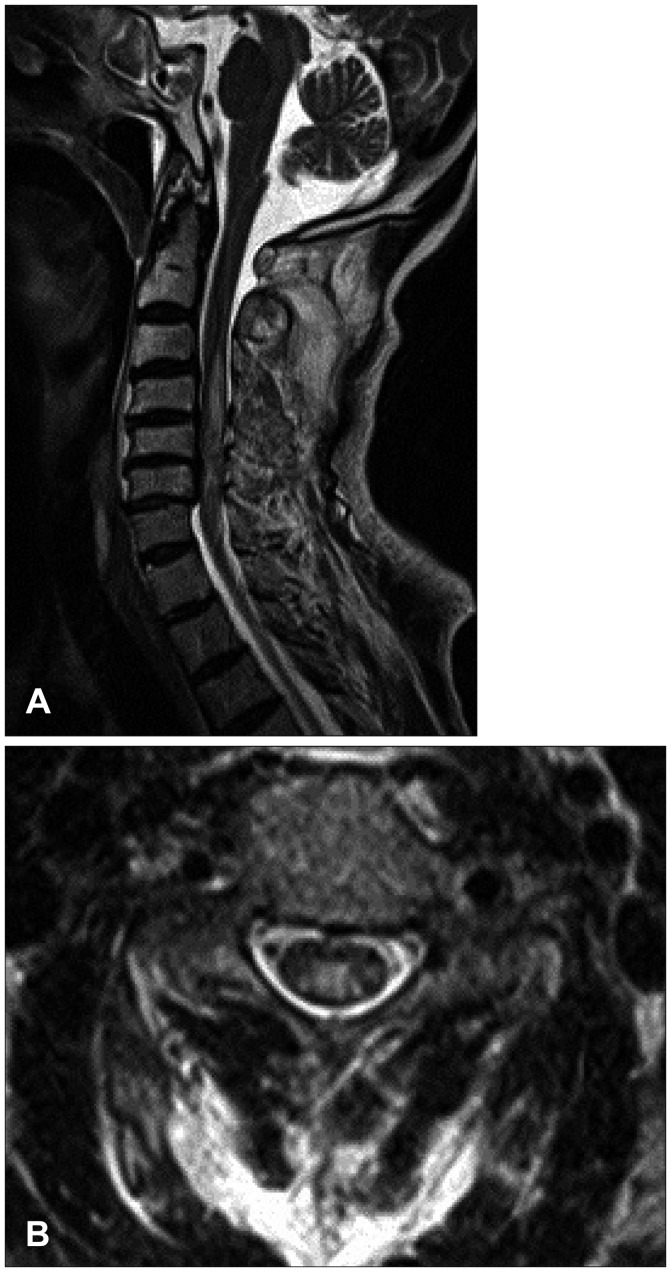
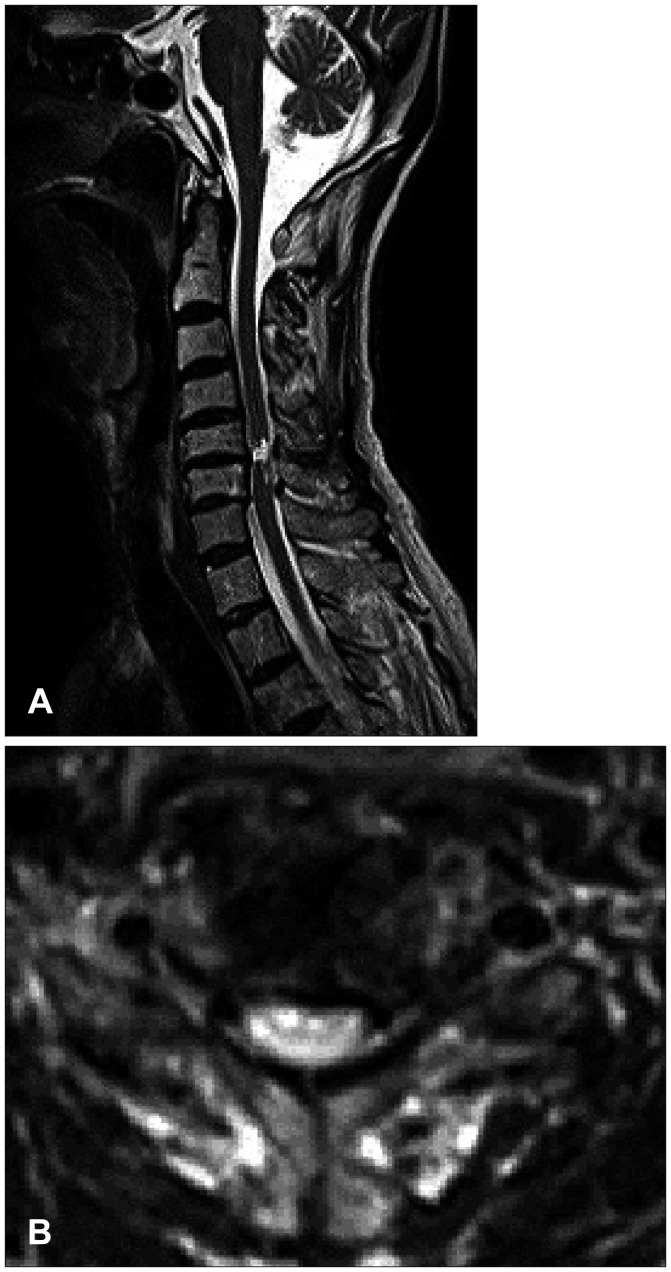
- We report a rare case of a 71-year-old male patient who had suffered from long-lasting neurogenic shock for 13 weeks after cervical spinal cord injury (SCI) caused by a bicycle accident. The neurogenic shock was resolved dramatically 2 weeks after the administration of alpha-1-adrenergic agonist, midodrine hydrochloride. In usual cases, neurogenic shock tends to improve between 2 and 6 weeks after SCI; however, in a few cases, the shock lasts for several months. In our case, spinal shock lasted for 13 weeks and exhibited very sensitive decline of blood pressure for even a slight decrease of dopamine despite recovered bulbospongiosus reflex. Three days after midodrine hydrochloride was added, hypotension improved dramatically. We discuss our rare case with pertinent literatures.
MeSH Terms
Figure
Reference
-
1. Barber DB, Rogers SJ, Fredrickson MD, Able AC. Midodrine hydrochloride and the treatment of orthostatic hypotension in tetraplegia: two cases and a review of the literature. Spinal Cord. 2000; 38:109–111. PMID: 10762185.
Article2. Casha S, Christie S. A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma. 2011; 28:1479–1495. PMID: 20030558.
Article3. Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med. 2008; 358:615–624. PMID: 18256396.4. Hagen EM, Faerestrand S, Hoff JM, Rekand T, Gronning M. Cardiovascular and urological dysfunction in spinal cord injury. Acta Neurol Scand Suppl. 2011; (191):71–78. PMID: 21711260.
Article5. McTavish D, Goa KL. Midodrine. A review of its pharmacological properties and therapeutic use in orthostatic hypotension and secondary hypotensive disorders. Drugs. 1989; 38:757–777. PMID: 2480881.6. Mukand J, Karlin L, Barrs K, Lublin P. Midodrine for the management of orthostatic hypotension in patients with spinal cord injury: a case report. Arch Phys Med Rehabil. 2001; 82:694–696. PMID: 11346851.
Article7. Nieshoff EC, Birk TJ, Birk CA, Hinderer SR, Yavuzer G. Double-blinded, placebo-controlled trial of midodrine for exercise performance enhancement in tetraplegia: a pilot study. J Spinal Cord Med. 2004; 27:219–225. PMID: 15478524.8. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000; 81:506–516. PMID: 10768544.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Midodrine for the Treatment of Hypotension in a Tetraplegic Patient with Cervical Cord Injury in ICU: A case report
- The Effect of Midodrine on Exercise-induced Hypotension in Cervical Cord Injury Patients
- Spinal Cord Injury Rehabilitation (II): Management of Neurogenic Bladder
- Urethral Stent as a Part of Management of the Neurogenic Bladder in Spinal Cord Injury: Two cases report
- Acute Spinal Cord Injury after Cervical Nerve Root Block