Cancer Res Treat.
2017 Jul;49(3):569-577. 10.4143/crt.2016.289.
An Open-Label, Randomized, Parallel, Phase III Trial Evaluating the Efficacy and Safety of Polymeric Micelle-Formulated Paclitaxel Compared to Conventional Cremophor EL-Based Paclitaxel for Recurrent or Metastatic HER2-Negative Breast Cancer
- Affiliations
-
- 1Center for Breast Cancer, National Cancer Center, Goyang, Korea. jungsilro@hotmail.com
- 2Department of Oncology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Division of Hematology-Oncology, Pusan National University Hospital, Busan, Korea.
- 5Medical Oncology Center, Seoul National University Hospital, Seoul, Korea.
- 6Division of Hematology-Oncology, Gachon University Gil Medical Center, Incheon, Korea.
- 7Division of Hematology-Oncology, Kosin University Gospel Hospital, Busan, Korea.
- 8Division of HematologyOncology, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 9Department of Hematology-Oncology, Korea University Guro Hospital, Seoul, Korea.
- 10Division of HematologyOncology, Daegu Catholic University Medical Center, Daegu, Korea.
- 11Division of Hematology-Oncology, Daegu Fatima Hospital, Daegu, Korea.
- 12Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 13Division of Hematology-Oncology, Inha University Hospital, Incheon, Korea.
- 14Division of Hematology-Oncology, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 15Division of Hematology-Oncology, Kyung Hee University Medical Center, Seoul, Korea.
- 16Samyang Biopharmaceuticals Corporation, Seoul, Korea.
- KMID: 2388302
- DOI: http://doi.org/10.4143/crt.2016.289
Abstract
- PURPOSE
Genexol-PM is a Cremophor EL-free formulation of low-molecular-weight, non-toxic, and biodegradable polymeric micelle-bound paclitaxel. We conducted a phase III study comparing the clinical efficacy and toxicity of Genexol-PM with conventional paclitaxel (Genexol).
MATERIALS AND METHODS
Patients were randomly assigned (1:1) to receive Genexol-PM 260 mg/m² or Genexol 175 mg/m² intravenously every 3 weeks. The primary outcome was the objective response rate (ORR).
RESULTS
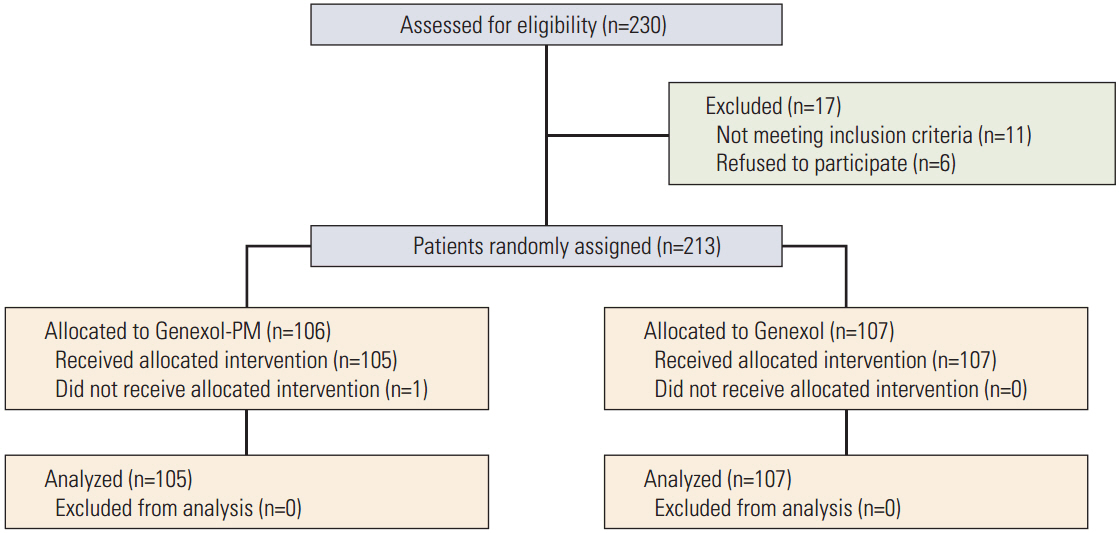
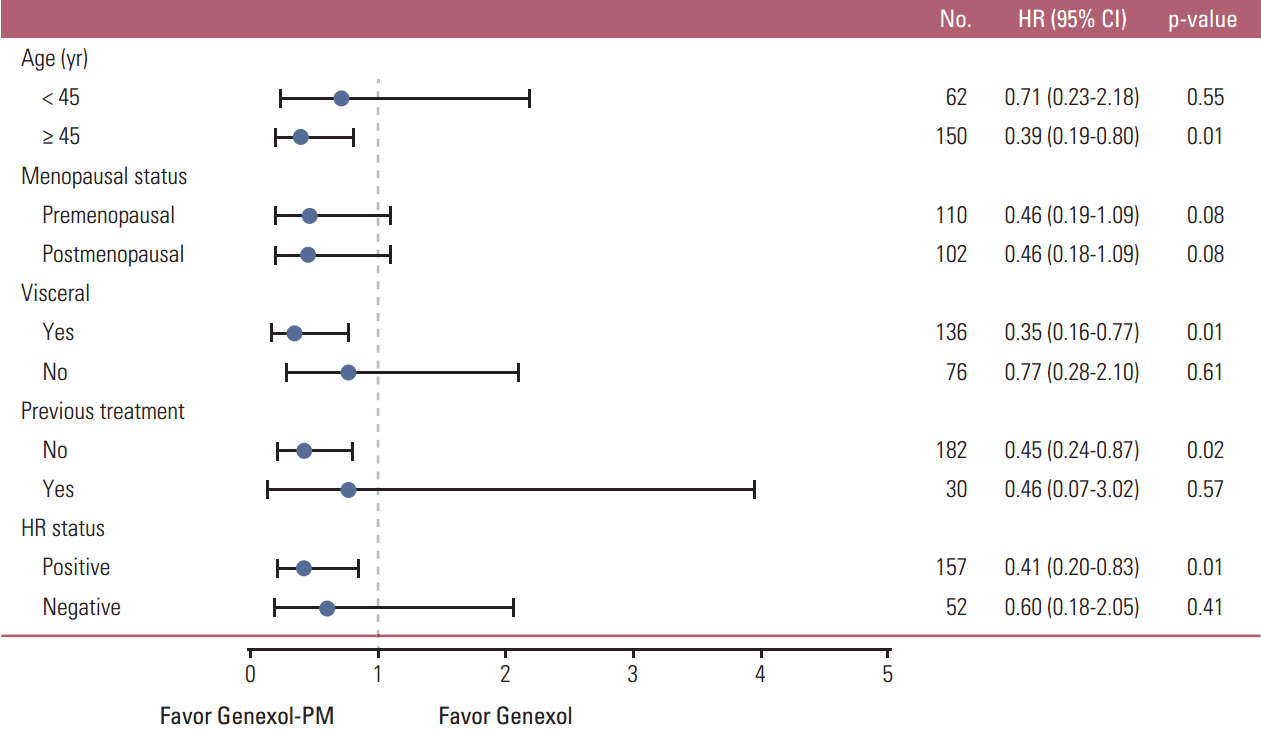
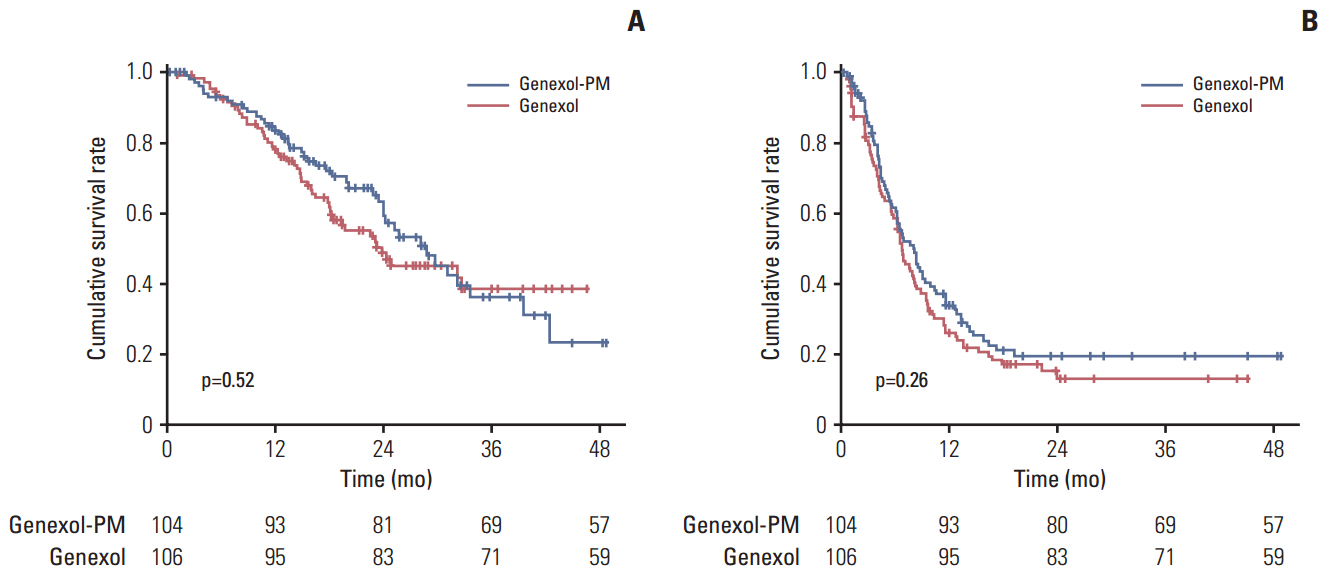
The study enrolled 212 patients, of whom 105 were allocated to receive Genexol-PM. The mean received dose intensity of Genexol-PM was 246.8±21.3 mg/m² (95.0%), and that of Genexol was 168.3±10.6 mg/m² (96.2%). After a median follow-up of 24.5 months (range, 0.0 to 48.7 months), the ORR of Genexol-PM was 39.1% (95% confidence interval [CI], 31.2 to 46.9) and the ORR of Genexol was 24.3% (95% CI, 17.5 to 31.1) (p(non-inferiority)=0.021, p(superiority)=0.016). The two groups did not differ significantly in overall survival (28.8 months for Genexol-PM vs. 23.8 months for Genexol; p=0.52) or progression-free survival (8.0 months for Genexol-PM vs. 6.7 months for Genexol; p=0.26). In both groups, the most common toxicities were neutropenia, with 68.6% occurrence in the Genexol-PM group versus 40.2% in the Genexol group (p < 0.01). The incidences of peripheral neuropathy of greater than grade 2 did not differ significantly between study treatments.
CONCLUSION
Compared with standard paclitaxel, Genexol-PM demonstrated non-inferior and even superior clinical efficacy with a manageable safety profile in patients with metastatic breast cancer.
MeSH Terms
Figure
Reference
-
References
1. Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008; 108:241–50.
Article2. Bernard-Marty C, Cardoso F, Piccart MJ. Use and abuse of taxanes in the management of metastatic breast cancer. Eur J Cancer. 2003; 39:1978–89.
Article3. Mathew AE, Mejillano MR, Nath JP, Himes RH, Stella VJ. Synthesis and evaluation of some water-soluble prodrugs and derivatives of taxol with antitumor activity. J Med Chem. 1992; 35:145–51.
Article4. Zhou HB, Zhu JR. Paclitaxel induces apoptosis in human gastric carcinoma cells. World J Gastroenterol. 2003; 9:442–5.
Article5. Nonaka M, Ikeda H, Fujisawa A, Uehara M, Inokuchi T. Induction of apoptosis by paclitaxel in human oral carcinoma cells. Int J Oral Maxillofac Surg. 2006; 35:649–52.
Article6. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995; 332:1004–14.
Article7. Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH. Nonlinear pharmacokinetics of paclitaxel in mice results from the pharmaceutical vehicle Cremophor EL. Cancer Res. 1996; 56:2112–5.8. Sparreboom A, van Zuylen L, Brouwer E, Loos WJ, de Bruijn P, Gelderblom H, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999; 59:1454–7.9. Crown J, O'Leary M. The taxanes: an update. Lancet. 2000; 355:1176–8.
Article10. Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001; 37:1590–8.11. van Zuylen L, Karlsson MO, Verweij J, Brouwer E, de Bruijn P, Nooter K, et al. Pharmacokinetic modeling of paclitaxel encapsulation in Cremophor EL micelles. Cancer Chemother Pharmacol. 2001; 47:309–18.
Article12. van Zuylen L, Verweij J, Sparreboom A. Role of formulation vehicles in taxane pharmacology. Invest New Drugs. 2001; 19:125–41.13. Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001; 72:191–202.
Article14. Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004; 10:3708–16.
Article15. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.16. Winer EP, Berry DA, Woolf S, Duggan D, Kornblith A, Harris LN, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: cancer and leukemia group B trial 9342. J Clin Oncol. 2004; 22:2061–8.
Article17. Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005; 23:7794–803.
Article18. Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Leung E, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nabpaclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol. 2015; 33:2361–9.
Article19. Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016; 17:345–56.
Article20. Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008; 26:1642–9.
Article21. Gradishar W, Krasnojon D, Cheporov S, Makhson A, Manikhas G, Clawson A, et al. Randomized comparison of nab-paclitaxel weekly or every 3 weeks compared to docetaxel every 3 weeks as first-line therapy in patients (pts) with metastatic breast cancer (MBC). Eur J Cancer. 2008; 6 Supple:172.
Article22. National Institute for Health and Care Excellence. NICE publishes final drug recommendations for the treatment of 3 separate medical conditions [Internet]. London: National Institute for Health and Care Excellence;2015. [cited 2015 Oct 28]. Available from: https://www.nice.org.uk/news/press-and-media/nice-publishes-final-drug-recommendations-for-the-treatment-of-3-separate-medical-conditions.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Non-convulsive seizure related to Cremophor ELâ„¢-free, polymeric micelle formulation of paclitaxel: a case report
- An Open-Label, Randomized, Parallel, Phase II Trial to Evaluate the Efficacy and Safety of a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel as First-Line Treatment for Ovarian Cancer: A Korean Gynecologic Oncology Group Study (KGOG-3021)
- A Case of Ischemic Colitis Associated with Paclitaxel Loaded Polymeric Micelle (Genexol-PM(R)) Chemotherapy
- Clinical Trial Protocol for ROSELLA: a phase 3 study of relacorilant in combination with nab-paclitaxel versus nab-paclitaxel monotherapy in advanced platinum-resistant ovarian cancer
- QL1604 plus paclitaxel-cisplatin/ carboplatin in patients with recurrent or metastatic cervical cancer: an open-label, single-arm, phase II trial