Cancer Res Treat.
2018 Jan;50(1):195-203. 10.4143/crt.2016.376.
An Open-Label, Randomized, Parallel, Phase II Trial to Evaluate the Efficacy and Safety of a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel as First-Line Treatment for Ovarian Cancer: A Korean Gynecologic Oncology Group Study (KGOG-3021)
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ymkim@amc.seoul.kr
- 2Department of Obstetrics and Gynecology, Keimyung University School of Medicine, Daegu, Korea.
- 3Department of Obstetrics and Gynecology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, Chonnam National University Medical School, Gwangju, Korea.
- 5Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 6Department of Obstetrics and Gynecology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 7Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University, Seoul, Korea.
- 8Department of Obstetrics and Gynecology, Ewha Womans University Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea.
- 9Department of Obstetrics and Gynecology, Ajou University School of Medicine, Suwon, Korea.
- 10Department of Obstetrics and Gynecology, Konkuk University Medical Center, Seoul, Korea.
- KMID: 2403489
- DOI: http://doi.org/10.4143/crt.2016.376
Abstract
- PURPOSE
Genexol-PM is a biodegradable cremophor EL-free polymeric micelle formulation of paclitaxel. Here,we compared efficacy and safety of Genexol-PM plus carboplatin versus Genexol plus carboplatin for ovarian cancer treatment.
MATERIALS AND METHODS
In this multicenter, randomized, phase II study, patients with International Federation of Gynecology and Obstetrics IC-IV epithelial ovarian cancer were randomly assigned (1:1) to receive Genexol-PM 260 mg/m2 or Genexol 175 mg/m2 with 5 area under the curve carboplatin every 3weeks (6 cycles). The primary endpointwas the carbohydrate antigen 125 and Response Evaluation Criteria In Solid Tumor composite overall response rate (ORR).
RESULTS
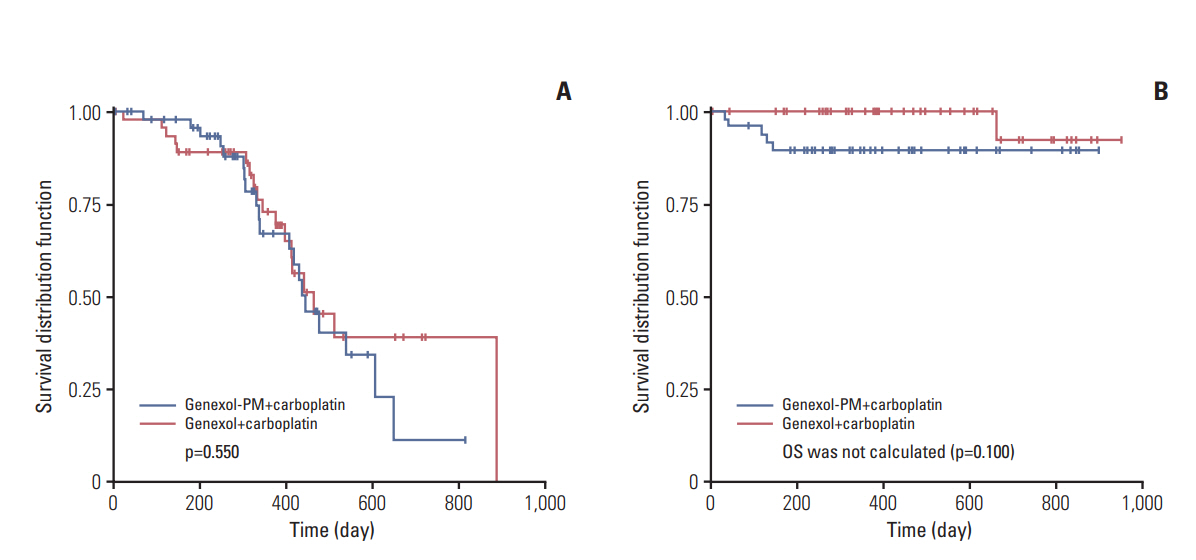
Of 131 enrolled patients, 98 were included in intention-to-treat analysis. Mean dosages were 260.00±0.00 mg/m2 Genexol-PM or 174.24±3.81 mg/m2 Genexol. Median followup was 18.0 months (range, 6.1 to 33.8 months). ORR was 88.0% (95% confidence interval [CI], 80.4 to 95.6) with Genexol-PM, and 77.1% (95% CI, 67.1 to 87.1) with Genexol (noninferiority threshold, 16.3%). Median time to progression was 14.8 months (95% CI, 11.3 to 20.2) with Genexol-PM and 15.4 months (95% CI, 13.2 to 29.6) with Genexol (p=0.550). Overall, six patients died. Neutropenia was the most common toxicity (incidences of 86.0% vs. 77.1%, p=0.120). Peripheral neuropathy incidences were 84.0% versus 64.6% (p= 0.148). Peripheral neuropathy of ≥ grade 3 occurred in one patient receiving Genexol. All toxicities were manageable.
CONCLUSION
Genexol-PM plus carboplatin as first-line treatment in patients with epithelial ovarian cancer demonstrated non-inferior efficacy and well-tolerated toxicities compared with the standard paclitaxel regimen. Further studies are warranted to optimize the dose and schedule, and to investigate long-term outcomes.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Safety and Tolerability of Weekly Genexol-PM, a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel, with Carboplatin in Gynecologic Cancer: A Phase I Study
So Hyun Nam, Shin-Wha Lee, Young-Jae Lee, Yong Man Kim
Cancer Res Treat. 2023;55(4):1346-1354. doi: 10.4143/crt.2022.1436.
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2012. Goyang: National Cancer Center;2014.3. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003; 21:3194–200.
Article4. Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011; 365:2473–83.
Article5. Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013; 14:1020–6.
Article6. Chan JK, Brady MF, Penson RT, Huang H, Birrer MJ, Walker JL, et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med. 2016; 374:738–48.
Article7. Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001; 37:1590–8.8. Kintzel PE. Prophylaxis for paclitaxel hypersensitivity reactions. Ann Pharmacother. 2001; 35:1114–7.
Article9. Kloover JS, den Bakker MA, Gelderblom H, van Meerbeeck JP. Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of premedication regimens. Br J Cancer. 2004; 90:304–5.
Article10. Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001; 72:191–202.
Article11. Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004; 10:3708–16.
Article12. Abstracts of the 43rd Annual Meeting of the Society of Gynecologic Oncology. March 24-27, 2012. Austin, Texas, USA. Gynecol Oncol. 2012; 125 Suppl 1:S2–188.13. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.14. Rustin GJ. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol. 2003; 21(10 Suppl):187s–93s.
Article15. Crown J, O’Leary M. The taxanes: an update. Lancet. 2000; 355:1176–8.
Article16. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995; 332:1004–14.
Article17. Sparreboom A, van Zuylen L, Brouwer E, Loos WJ, de Bruijn P, Gelderblom H, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999; 59:1454–7.18. van Zuylen L, Karlsson MO, Verweij J, Brouwer E, de Bruijn P, Nooter K, et al. Pharmacokinetic modeling of paclitaxel encapsulation in Cremophor EL micelles. Cancer Chemother Pharmacol. 2001; 47:309–18.
Article19. Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008; 108:241–50.
Article20. Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophorfree, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007; 18:2009–14.
Article21. Lee JL, Ahn JH, Park SH, Lim HY, Kwon JH, Ahn S, et al. Phase II study of a cremophor-free, polymeric micelle formulation of paclitaxel for patients with advanced urothelial cancer previously treated with gemcitabine and platinum. Invest New Drugs. 2012; 30:1984–90.
Article22. Saif MW, Podoltsev NA, Rubin MS, Figueroa JA, Lee MY, Kwon J, et al. Phase II clinical trial of paclitaxel loaded polymeric micelle in patients with advanced pancreatic cancer. Cancer Invest. 2010; 28:186–94.
Article23. Winer EP, Berry DA, Woolf S, Duggan D, Kornblith A, Harris LN, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: cancer and leukemia group B trial 9342. J Clin Oncol. 2004; 22:2061–8.
Article24. Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005; 23:7794–803.
Article25. Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012; 30:2055–62.
Article26. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013; 369:1691–703.
Article27. Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Leung E, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound Nabpaclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol. 2015; 33:2361–9.
Article28. Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016; 17:345–56.
Article29. Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol. 2006; 4:165–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Non-convulsive seizure related to Cremophor ELâ„¢-free, polymeric micelle formulation of paclitaxel: a case report
- An open-label, multicenter, phase I trial of a cremophor-free, polymeric micelle formulation of paclitaxel combined with carboplatin as a first-line treatment for advanced ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG-3016)
- Safety and Tolerability of Weekly Genexol-PM, a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel, with Carboplatin in Gynecologic Cancer: A Phase I Study
- An Open-Label, Randomized, Parallel, Phase III Trial Evaluating the Efficacy and Safety of Polymeric Micelle-Formulated Paclitaxel Compared to Conventional Cremophor EL-Based Paclitaxel for Recurrent or Metastatic HER2-Negative Breast Cancer
- Clinical Trial Protocol for ROSELLA: a phase 3 study of relacorilant in combination with nab-paclitaxel versus nab-paclitaxel monotherapy in advanced platinum-resistant ovarian cancer