Immune Netw.
2017 Aug;17(4):261-268. 10.4110/in.2017.17.4.261.
Localization of Serum Amyloid A3 in the Mouse Ovary
- Affiliations
-
- 1Department of Biochemistry and Cancer Biology, Meharry Medical College, Nashville, TN 37208, USA. dson@mmc.edu
- 2Department of Pharmaceutical Sciences, College of Pharmacy, Florida A&M University, Tallahassee, FL 32301, USA.
- 3Center for Reproductive Sciences, University of Kansas Medical Center, Kansas City, KS 66160, USA.
- 4Department of Anatomy and Cell Biology, University of Kansas Medical Center, Kansas City, KS 66160, USA.
- 5Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS 66160, USA.
- 6Department of Obstetrics and Gynecology, University of Kansas Medical Center, Kansas City, KS 66160, USA.
- KMID: 2388080
- DOI: http://doi.org/10.4110/in.2017.17.4.261
Abstract
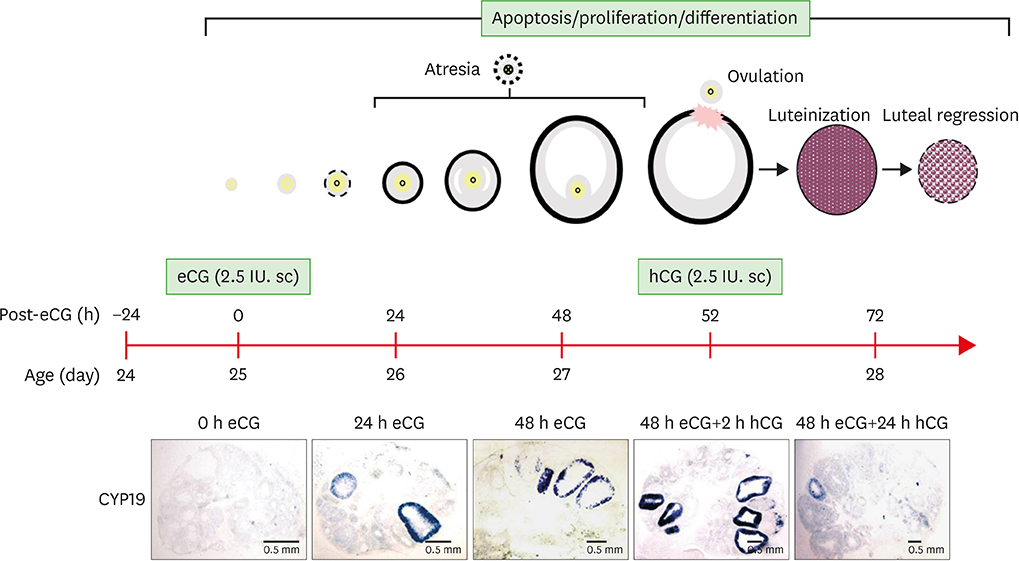
- Tumor necrosis factor-α (TNF-α) induces serum amyloid A (SAA) 3 among acute-phase proteins in mouse granulosa cells by activating NF-κB signaling via p55 TNF-α receptor type 1. However, the localization of SAA3 within the ovary is unknown. Here we investigated ovarian localization of SAA3 in a mouse ovulation model and in response to IL-1β, a proinflammatory mediator. For the ovulation model, equine chorionic gonadotropin (eCG; 2.5 IU) was administered to mice subcutaneously (sc) to stimulate follicular development on day 25 of age and then 50 h after eCG, human chorionic gonadotropin (hCG; 2.5 IU) was administered sc to induce ovulation. The mouse ovulation model was characterized by the localization of CYP19 mRNA expression to granulosa layers of larger follicles. SAA3 mRNA, determined by in situ hybridization, was broadly expressed throughout the whole ovary. Granulosa layers and small follicles expressed higher SAA3 mRNA compared to thecal-interstitial layers and large follicles, respectively. Interestingly, atretic follicles contained cells expressing intense SAA3 mRNA. After ovulation, SAA3 mRNA expression was intensely evident in ruptured follicles and corpora lutea (CL). The intraperitoneal administration of IL-1β revealed the intense and extensive appearance of specific cells expressing SAA3 mRNA around follicles and in CL. In addition, Gene Expression Omnibus (GEO) database analysis supported expression pattern of SAA3 mRNA observed in mouse ovulation model. Taken together, SAA3 was broadly distributed through the whole ovary, but intensely expressed in atretic follicles and CL. Furthermore, proinflammatory mediators could trigger the intense appearance of SAA3 around follicles and in CL.
Keyword
MeSH Terms
-
Acute-Phase Proteins
Amyloid*
Animals
Aromatase
Chorionic Gonadotropin
Corpus Luteum
Electrocardiography
Female
Gene Expression
Granulosa Cells
In Situ Hybridization
Mice*
Necrosis
Ovarian Follicle
Ovary*
Ovulation
RNA, Messenger
Serum Amyloid A Protein
Acute-Phase Proteins
Amyloid
Aromatase
Chorionic Gonadotropin
RNA, Messenger
Serum Amyloid A Protein
Figure
Reference
-
1. Targońska-Stępniak B, Majdan M. Serum amyloid A as a marker of persistent inflammation and an indicator of cardiovascular and renal involvement in patients with rheumatoid arthritis. Mediators Inflamm. 2014; 2014:793628.
Article2. Urieli-Shoval S, Cohen P, Eisenberg S, Matzner Y. Widespread expression of serum amyloid A in histologically normal human tissues. Predominant localization to the epithelium. J Histochem Cytochem. 1998; 46:1377–1384.
Article3. Urieli-Shoval S, Finci-Yeheskel Z, Dishon S, Galinsky D, Linke RP, Ariel I, Levin M, Ben-Shachar I, Prus D. Expression of serum amyloid a in human ovarian epithelial tumors: implication for a role in ovarian tumorigenesis. J Histochem Cytochem. 2010; 58:1015–1023.
Article4. Meek RL, Urieli-Shoval S, Benditt EP. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc Natl Acad Sci USA. 1994; 91:3186–3190.
Article5. Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010; 2010:535918.
Article6. Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006; 3:e287.
Article7. Son DS, Terranova PF, Roby KF. Interaction of adenosine 3′,5′-cyclic monophosphate and tumor necrosis factor-alpha on serum amyloid A3 expression in mouse granulosa cells: dependence on CCAAT-enhancing binding protein-beta isoform. Endocrinology. 2010; 151:3407–3419.
Article8. Takase H, Tanaka M, Miyagawa S, Yamada T, Mukai T. Effect of amino acid variations in the central region of human serum amyloid A on the amyloidogenic properties. Biochem Biophys Res Commun. 2014; 444:92–97.
Article9. Uhlar CM, Burgess CJ, Sharp PM, Whitehead AS. Evolution of the serum amyloid A (SAA) protein superfamily. Genomics. 1994; 19:228–235.
Article10. Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999; 265:501–523.
Article11. Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009; 138:903–919.
Article12. Espey LL. Ovulation as an inflammatory reaction--a hypothesis. Biol Reprod. 1980; 22:73–106.
Article13. Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994; 50:233–238.
Article14. Gaytán M, Morales C, Bellido C, Sánchez-Criado JE, Gaytán F. Non-steroidal anti-inflammatory drugs (NSAIDs) and ovulation: lessons from morphology. Histol Histopathol. 2006; 21:541–556.15. Son DS, Roby KF, Terranova PF. Tumor necrosis factor-alpha induces serum amyloid A3 in mouse granulosa cells. Endocrinology. 2004; 145:2245–2252.
Article16. Ghersevich S, Isomaa V, Vihko P. Cytokine regulation of the expression of estrogenic biosynthetic enzymes in cultured rat granulosa cells. Mol Cell Endocrinol. 2001; 172:21–30.
Article17. Nakayama M, Manabe N, Inoue N, Matsui T, Miyamoto H. Changes in the expression of tumor necrosis factor (TNF) alpha, TNFalpha receptor (TNFR) 2, and TNFR-associated factor 2 in granulosa cells during atresia in pig ovaries. Biol Reprod. 2003; 68:530–535.
Article18. Brännström M, Bonello N, Wang LJ, Norman RJ. Effects of tumour necrosis factor alpha (TNF alpha) on ovulation in the rat ovary. Reprod Fertil Dev. 1995; 7:67–73.
Article19. Roby KF, Son DS, Terranova PF. Alterations of events related to ovarian function in tumor necrosis factor receptor type I knockout mice. Biol Reprod. 1999; 61:1616–1621.
Article20. Son DS, Roby KF. Interleukin-1alpha-induced chemokines in mouse granulosa cells: impact on keratinocyte chemoattractant chemokine, a CXC subfamily. Mol Endocrinol. 2006; 20:2999–3013.
Article21. Park-Sarge OK, Mayo KE. Regulation of the progesterone receptor gene by gonadotropins and cyclic adenosine 3′,5′-monophosphate in rat granulosa cells. Endocrinology. 1994; 134:709–718.
Article22. Perez-Llamas C, Lopez-Bigas N. Gitools: analysis and visualisation of genomic data using interactive heat-maps. PLoS One. 2011; 6:e19541.
Article23. Ignacio RM, Kabir SM, Lee ES, Adunyah SE, Son DS. NF-κB-mediated CCL20 reigns dominantly in CXCR2-driven ovarian cancer progression. PLoS One. 2016; 11:e0164189.
Article24. Fitzpatrick SL, Carlone DL, Robker RL, Richards JS. Expression of aromatase in the ovary: down-regulation of mRNA by the ovulatory luteinizing hormone surge. Steroids. 1997; 62:197–206.
Article25. Inaoka Y, Yazawa T, Mizutani T, Kokame K, Kangawa K, Uesaka M, Umezawa A, Miyamoto K. Regulation of P450 oxidoreductase by gonadotropins in rat ovary and its effect on estrogen production. Reprod Biol Endocrinol. 2008; 6:62.
Article26. Kaipia A, Hsueh AJ. Regulation of ovarian follicle atresia. Annu Rev Physiol. 1997; 59:349–363.
Article27. Gadsby J, Rose L, Sriperumbudur R, Ge Z. The role of intra-luteal factors in the control of the porcine corpus luteum. Soc Reprod Fertil Suppl. 2006; 62:69–83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Macroglossia due to Amyloidosis Associated with Multiple Myeloma
- Secondary renal amyloidosis in a 13-year-old girl with bronchiectasis
- In vivo Image of Cerebral Amyloid Angiopathy in an Alzheimer's Disease Mouse Model
- Reactive Oxygen Species Production, Expression of Complement Regulator Genes and Phagocytosis in the Murine Microglial Cell after Administration of Beta-Amyloid(A beta1-42) Protein
- Secondary Localized Cutaneous Amyloidosis in Seborrheic Keratosis