Korean J Physiol Pharmacol.
2017 Jul;21(4):385-395. 10.4196/kjpp.2017.21.4.385.
Elucidation of the profound antagonism of contractile action of phenylephrine in rat aorta effected by an atypical sympathomimetic decongestant
- Affiliations
-
- 1Department of Pharmacology, Faculty of Pharmacy, University of Belgrade, Vojvode Stepe 450, 11221 Belgrade, Serbia. miroslav@pharmacy.bg.ac.rs
- KMID: 2384452
- DOI: http://doi.org/10.4196/kjpp.2017.21.4.385
Abstract
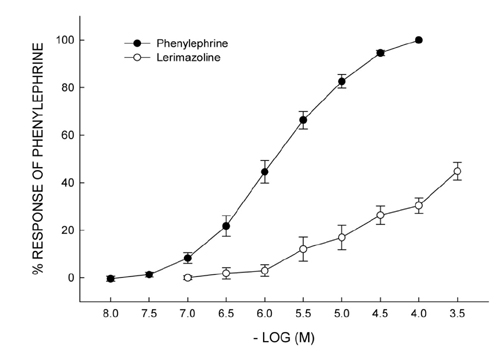
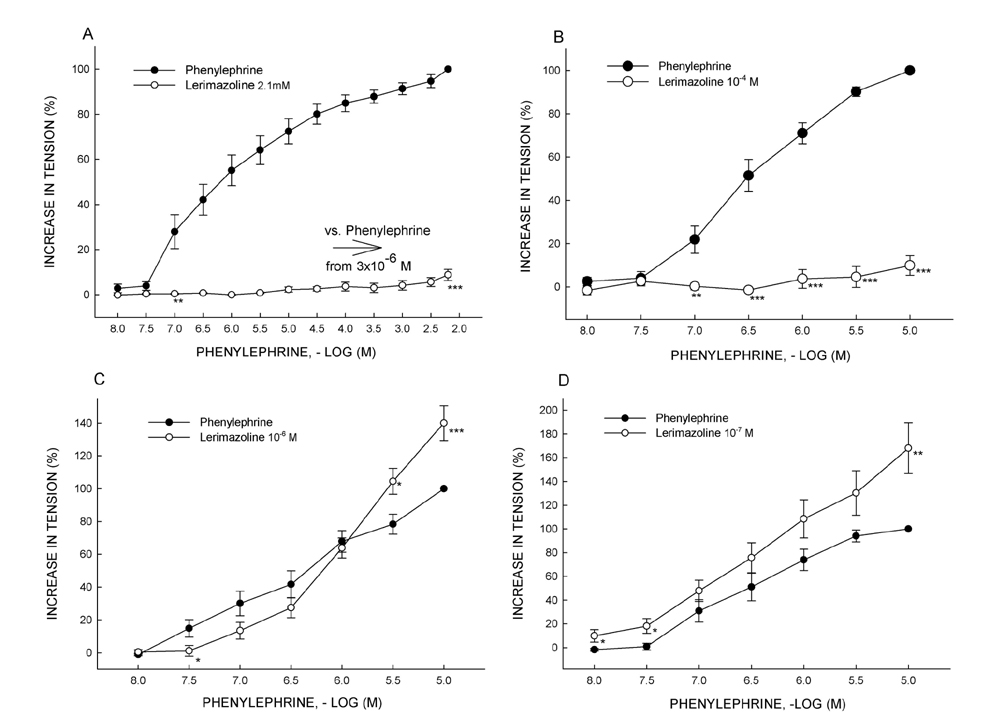
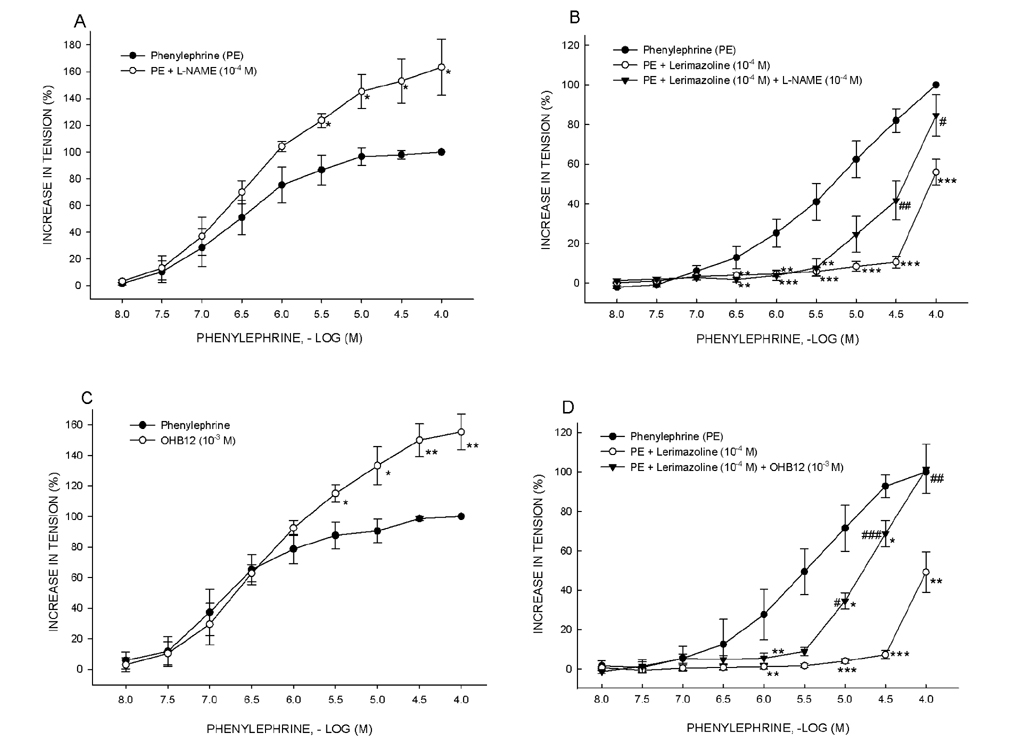
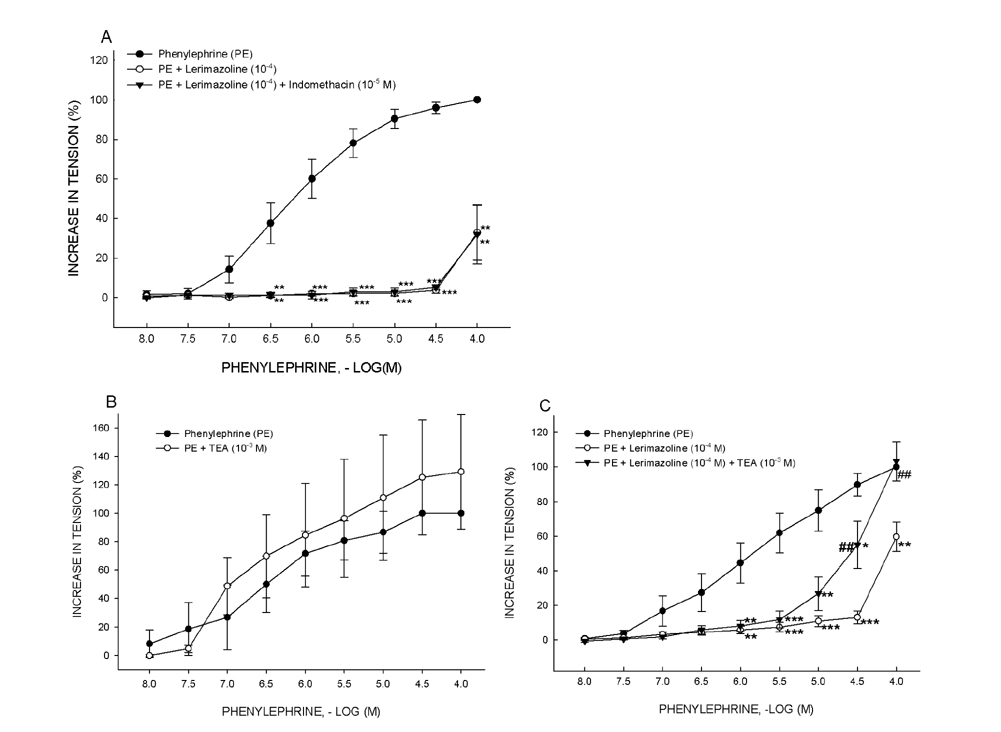
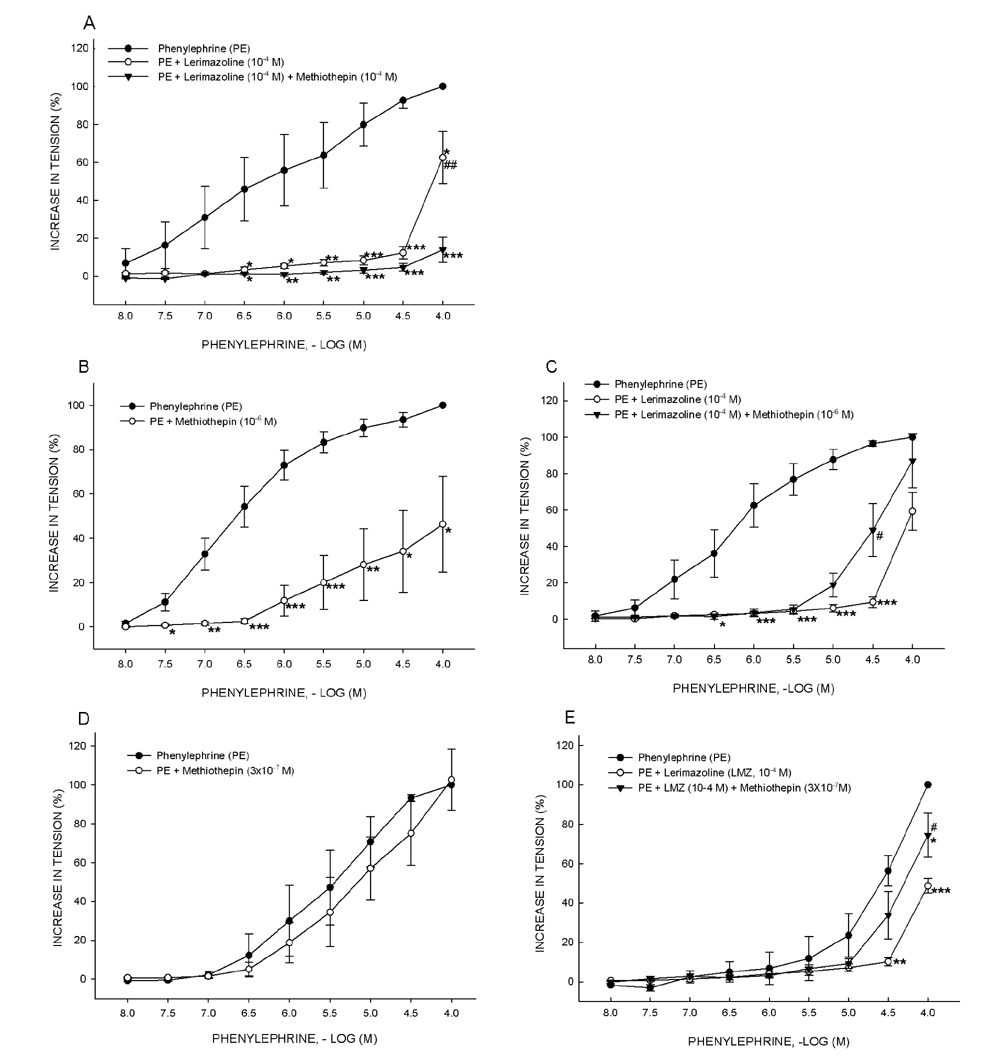
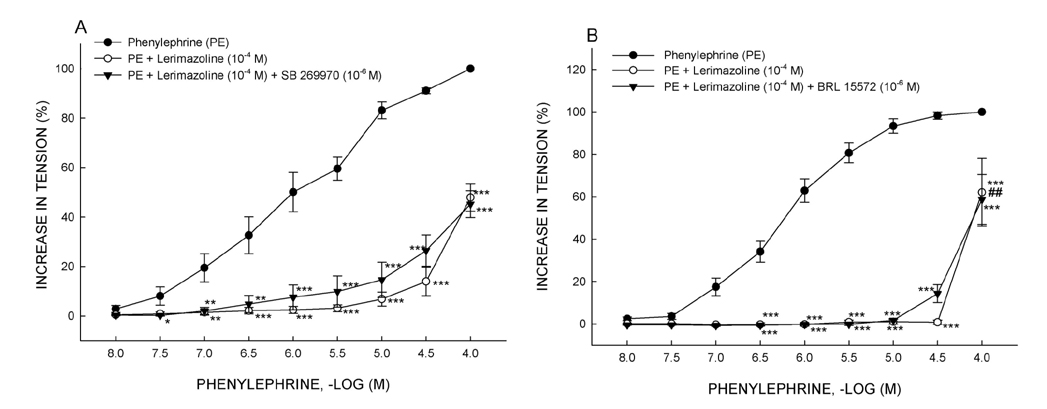
- Vasoconstrictive properties of sympathomimetic drugs are the basis of their widespread use as decongestants and possible source of adverse responses. Insufficiently substantiated practice of combining decongestants in some marketed preparations, such are those containing phenylephrine and lerimazoline, may affect the overall contractile activity, and thus their therapeutic utility. This study aimed to examine the interaction between lerimazoline and phenylephrine in isolated rat aortic rings, and also to assess the substrate of the obtained lerimazoline-induced attenuation of phenylephrine contraction. Namely, while lower concentrations of lerimazoline (10â»â¶ M and especially 10â»â· M) expectedly tended to potentiate the phenylephrine-induced contractions, lerimazoline in higher concentrations (10â»â´ M and above) unexpectedly and profoundly depleted the phenylephrine concentration-response curve. Suppression of NO with NO synthase (NOS) inhibitor N(w)-nitro-L-arginine methyl ester (L-NAME; 10â»â´ M) or NO scavanger OHBâ‚â‚‚ (10⻳ M), as well as non-specific inhibition of Kâº-channels with tetraethylammonium (TEA; 10⻳ M), have reversed lerimazoline-induced relaxation of phenylephrine contractions, while cyclooxygenase inhibitor indomethacin (10â»âµ M) did not affect the interaction between two vasoconstrictors. At the receptor level, non-selective 5-HT receptor antagonist methiothepin reversed the attenuating effect of lerimazoline on phenylephrine contraction when applied at 3×10â»â· and 10â»â¶ M, but not at the highest concentration (10â»â´ M). Neither the 5-HT1D-receptor selective antagonist BRL 15572 (10â»â¶ M) nor 5-HT₇ receptor selective antagonist SB 269970 (10â»â¶ M) affected the lerimazoline-induced attenuation of phenylephrine activity. The mechanism of lerimazoline-induced suppression of phenylephrine contractions may involve potentiation of activity of NO and Kâº-channels and activation of some methiothepin-sensitive receptors, possibly of the 5-HT(2B) subtype.
Keyword
MeSH Terms
-
Animals
Aorta*
Indomethacin
Methiothepin
Nasal Decongestants
Nitric Oxide Synthase
Phenylephrine*
Prostaglandin-Endoperoxide Synthases
Rats*
Relaxation
Serotonin
Sympathomimetics
Tetraethylammonium
Vasoconstrictor Agents
Indomethacin
Methiothepin
Nasal Decongestants
Nitric Oxide Synthase
Phenylephrine
Prostaglandin-Endoperoxide Synthases
Serotonin
Sympathomimetics
Tetraethylammonium
Vasoconstrictor Agents
Figure
Reference
-
1. Corey JP, Houser SM, Ng BA. Nasal congestion: a review of its etiology, evaluation, and treatment. Ear Nose Throat J. 2000; 79:690–698.2. Furukawa K, Chess-Williams R, Uchiyama T. Alpha 1B-adrenoceptor subtype mediating the phenylephrine-induced contractile response in rabbit corpus cavernosum penis. Jpn J Pharmacol. 1996; 71:325–331.3. Hussain MB, Marshall I. Characterization of alpha1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br J Pharmacol. 1997; 122:849–858.4. Morton JS, Daly CJ, Jackson VM, McGrath JC. Alpha(1A)-adrenoceptors mediate contractions to phenylephrine in rabbit penile arteries. Br J Pharmacol. 2007; 150:112–120.5. Brayfield A, Martindale W. Martindale: the complete drug reference. 38th ed. London: Pharmaceutical Press;2014.6. Boudier HS, de Boer J, Smeets G, Lien EJ, van Rossum J. Structure activity relationships for central and peripheral alpha adrenergic activities of imidazoline derivatives. Life Sci. 1975; 17:377–385.7. Malta E, Ong JS, Raper C, Tawa PE, Vaughan GN. Structure-activity relationships of clonidine- and tolazoline-like compounds at histamine and alpha-adrenoceptor sites. Br J Pharmacol. 1980; 69:679–688.8. Nathanson JA. Phenyliminoimidazolidines. Characterization of a class of potent agonists of octopamine-sensitive adenylate cyclase and their use in understanding the pharmacology of octopamine receptors. Mol Pharmacol. 1985; 28:254–268.9. Law H, Dukat M, Teitler M, Lee DK, Mazzocco L, Kamboj R, Rampersad V, Prisinzano T, Glennon RA. Benzylimidazolines as h5-HT1B/1D serotonin receptor ligands: a structure-affinity investigation. J Med Chem. 1998; 41:2243–2251.10. Bhattacharya A, Schenck KW, Xu YC, Nisenbaum L, Galbreath E, Cohen ML. 5-Hydroxytryptamine1B receptor-mediated contraction of rabbit saphenous vein and basilar artery: role of vascular endothelium. J Pharmacol Exp Ther. 2004; 309:825–832.11. Rameshrad M, Babaei H, Azarmi Y, Fouladia DF. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci. 2016; 145:190–204.12. Morán A, Ortiz de Urbina AV, Martín ML, García M, Rodriguez-Barbero A, Dorado F, San Román L. Characterization of contractile 5-hydroxytryptamine receptor subtypes in the in situ autoperfused kidney in the anaesthetized rat. Eur J Pharmacol. 2008; 592:133–137.13. Fernández MM, Morán A, Martín ML, San Román L. Mesenteric vasoconstrictor response to 5-hydroxytryptamine in the in situ blood autoperfused rat mesentery: involvement of 5-HT(2B) and/or 5-HT(2C) receptor activation. Eur J Pharmacol. 2000; 401:221–227.14. Calama E, Fernández MM, Morán A, Martín ML, San Román L. Vasodilator and vasoconstrictor responses induced by 5-hydroxytryptamine in the in situ blood autoperfused hindquarters of the anaesthetized rat. Naunyn Schmiedebergs Arch Pharmacol. 2002; 366:110–116.15. Flavahan NA, McGrath JC. Are human vascular alpha-adrenoceptors atypical? J Cardiovasc Pharmacol. 1984; 6:208–210.16. Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent alpha(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol. 2000; 278:H1075–H1083.17. Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980; 47:1–9.18. Jerez S, Peral de, Coviello A. Cross talk between angiotensin II and alpha 1 adrenergic receptors in rabbit aorta: role of endothelium. J Cardiovasc Pharmacol. 2004; 43:402–409.19. Lüscher TF. 1993 Mack Forster Award Lecture. Review. The endothelium as a target and mediator of cardiovascular disease. Eur J Clin Invest. 1993; 23:670–685.20. Marín J, Rodríguez-Martínez MA. Role of vascular nitric oxide in physiological and pathological conditions. Pharmacol Ther. 1997; 75:111–134.21. Hatake K, Wakabayashi I, Hishida S. Endothelium-dependent relaxation resistant to NG-nitro-L-arginine in rat aorta. Eur J Pharmacol. 1995; 274:25–32.22. Stallone JN. Role of endothelium in sexual dimorphism in vasopressin-induced contraction of rat aorta. Am J Physiol. 1993; 265:H2073–H2080.23. López-Canales JS, Lozano-Cuenca J, Muãoz-Islas E, Aguilar-Carrasco JC, López-Canales OA, López-Mayorga RM, Castillo-Henkel EF, Valencia-Hernández I, Castillo-Henkel C. Mechanisms involved in the vasorelaxant effects produced by the acute application of amfepramone in vitro to rat aortic rings. Braz J Med Biol Res. 2015; 48:537–544.24. Watts SW, Darios ES, Seitz BM, Thompson JM. 5-HT is a potent relaxant in rat superior mesenteric veins. Pharmacol Res Perspect. 2015; 3:e00103.25. Terrón JA, Falcón-Neri A. Pharmacological evidence for the 5-HT7 receptor mediating smooth muscle relaxation in canine cerebral arteries. Br J Pharmacol. 1999; 127:609–616.26. Muñoz-Islas E, Lozano-Cuenca J, González-Hernández A, Ramírez-Rosas MB, Sánchez-López A, Centurión D, Maassenvandenbrink A, Villalón CM. Spinal sumatriptan inhibits capsaicin-induced canine external carotid vasodilatation via 5-HT1B rather than 5-HT1D receptors. Eur J Pharmacol. 2009; 615:133–138.27. Ishida T, Kawashima S, Hirata K, Yokoyama M. Nitric oxide is produced via 5-HT1B and 5-HT2B receptor activation in human coronary artery endothelial cells. Kobe J Med Sci. 1998; 44:51–63.28. Borgdorff P, Fekkes D, Tangelder GJ. Hypotension caused by extracorporeal circulation: serotonin from pump-activated platelets triggers nitric oxide release. Circulation. 2002; 106:2588–2593.29. Lamarre NS, Raffa RB, Tallarida RJ. Cocaine synergism with α agonists in rat aorta: computational analysis reveals an action beyond reuptake inhibition. Drug Alcohol Depend. 2013; 129:226–231.30. Li W, Su J, Sehgal S, Altura BT, Altura BM. Cocaine-induced relaxation of isolated rat aortic rings and mechanisms of action: possible relation to cocaine-induced aortic dissection and hypotension. Eur J Pharmacol. 2004; 496:151–158.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Total Ginseng Saponin on Contractile Responses of Vasoconstrictors in the Isolated Rat Aorta
- Comparison of Vasodilator Effects of Platycodin D and D3 in Rats
- Effect of Ethanol on the Regulation of Smooth Muscle Tone in Rat Aorta
- The Infuluences of Sympathomimetic Amines on Melanophores of the Frog Skin
- Participation of COX-1 and COX-2 in the contractile effect of phenylephrine in prepubescent and old rats