J Gastric Cancer.
2017 Jun;17(2):132-144. 10.5230/jgc.2017.17.e16.
Prognostic Factor Analysis of Overall Survival in Gastric Cancer from Two Phase III Studies of Second-line Ramucirumab (REGARD and RAINBOW) Using Pooled Patient Data
- Affiliations
-
- 1Dana-Farber Cancer Institute, Boston, MA, USA. charles.fuchs@yale.edu
- 2Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
- 3Masaryk Memorial Cancer Institute, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
- 4University Hospital Gasthuisberg Leuven and KU Leuven, Leuven, Belgium.
- 5Gangnam Severance Hospital, Seoul, Korea.
- 6Korea University Guro Hospital, Seoul, Korea.
- 7Oncology Research Group, Brown University, Providence, RI, USA.
- 8Szent László Hospital, Budapest, Hungary.
- 9Royal Marsden Hospital, London and Surrey, United Kingdom.
- 10Gastrointestinal Medical Oncology Division, National Cancer Center Hospital, Tokyo, Japan.
- 11Institute of Clinical Research, Universitären Centrum für Tumorerkrankungen-University Cancer Center, Frankfurt, Germany.
- 12Istituti Ospitalieri di Cremona, Cremona, Italy.
- 13Exploratory Oncology Research and Clinical Trial Center, National Cancer Center, Kashiwa, Japan.
- 14Lilly Deutschland GmbH, Bad Homburg, Germany.
- 15Eli Lilly and Company, Bridgewater, NJ, USA.
- 16Eli Lilly and Company, Indianapolis, IN, USA.
- 17Departments of Oncology and Hematology with Integrated Palliative Care, Kliniken Essen-Mitte, Essen, Germany.
- KMID: 2383736
- DOI: http://doi.org/10.5230/jgc.2017.17.e16
Abstract
- PURPOSE
To identify baseline prognostic factors for survival in patients with disease progression, during or after chemotherapy for the treatment of advanced gastric or gastroesophageal junction (GEJ) cancer.
MATERIALS AND METHODS
We pooled data from patients randomized between 2009 and 2012 in 2 phase III, global double-blind studies of ramucirumab for the treatment of advanced gastric or GEJ adenocarcinoma following disease progression on first-line platinum- and/or fluoropyrimidine-containing therapy (REGARD and RAINBOW). Forty-one key baseline clinical and laboratory factors common in both studies were examined. Model building started with covariate screening using univariate Cox models (significance level=0.05). A stepwise multivariable Cox model identified the final prognostic factors (entry+exit significance level=0.01). Cox models were stratified by treatment and geographic region. The process was repeated to identify baseline prognostic quality of life (QoL) parameters.
RESULTS
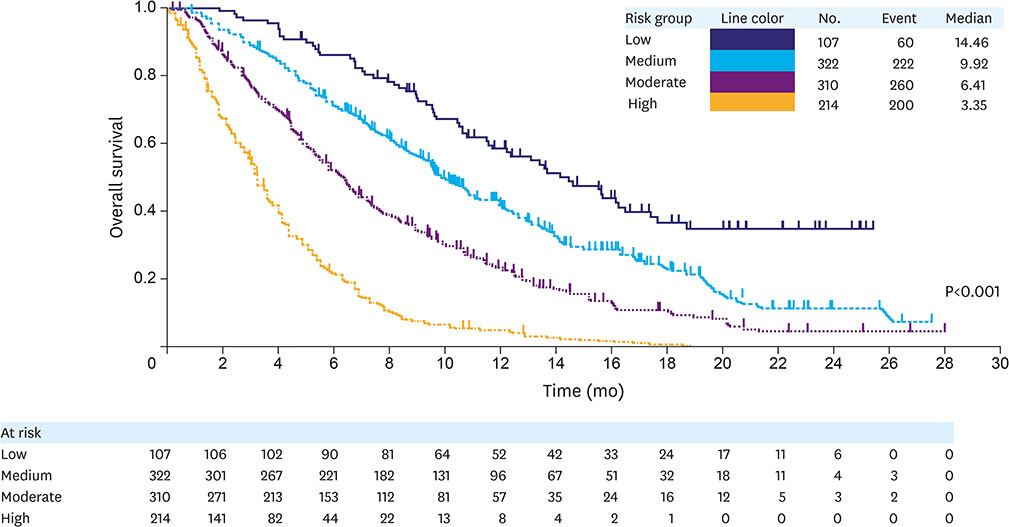
Of 1,020 randomized patients, 953 (93%) patients without any missing covariates were included in the analysis. We identified 12 independent prognostic factors of poor survival: 1) peritoneal metastases; 2) Eastern Cooperative Oncology Group (ECOG) performance score 1; 3) the presence of a primary tumor; 4) time to progression since prior therapy <6 months; 5) poor/unknown tumor differentiation; abnormally low blood levels of 6) albumin, 7) sodium, and/or 8) lymphocytes; and abnormally high blood levels of 9) neutrophils, 10) aspartate aminotransferase (AST), 11) alkaline phosphatase (ALP), and/or 12) lactate dehydrogenase (LDH). Factors were used to devise a 4-tier prognostic index (median overall survival [OS] by risk [months]: high=3.4, moderate=6.4, medium=9.9, and low=14.5; Harrell's C-index=0.66; 95% confidence interval [CI], 0.64-0.68). Addition of QoL to the model identified patient-reported appetite loss as an independent prognostic factor.
CONCLUSIONS
The identified prognostic factors and the reported prognostic index may help clinical decision-making, patient stratification, and planning of future clinical studies.
MeSH Terms
-
Adenocarcinoma
Alkaline Phosphatase
Appetite
Aspartate Aminotransferases
Clinical Decision-Making
Disease Progression
Double-Blind Method
Drug Therapy
Esophagogastric Junction
Factor Analysis, Statistical*
Humans
L-Lactate Dehydrogenase
Lymphocytes
Mass Screening
Neoplasm Metastasis
Neutrophils
Prognosis
Proportional Hazards Models
Quality of Life
Sodium
Stomach Neoplasms*
Alkaline Phosphatase
Aspartate Aminotransferases
L-Lactate Dehydrogenase
Sodium
Figure
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.2. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon: International Agency for Research on Cancer;cited 2015 Aug 1. Available from: http://globocan.iarc.fr.3. Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, editors. SEER Cancer Statistics Review, 1975??012 (based on November 2014 SEER data submission, posted to the SEER web site, April 2015) [Internet]. Bethesda (MD): National Cancer Institute;2015. cited 2015 Aug 1. Available from: http://seer.cancer.gov/csr/1975_2012/.4. Avital I, Pisters PW, Kelsen DP, Willett CG. Cancer of the stomach. In : DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia (PA): Lippincott Williams & Wilkins;2011. p. 924–954.5. Koh TJ, Wang TC. Tumors of the stomach. In : Feldman M, Friedman LS, Sleisenger MH, editors. Sleisenger & Fordtran's Gastrointestinal and Liver Disease. 7th ed. Philadelphia (PA): Saunders;2002. p. 829–844.6. Layke JC, Lopez PP. Gastric cancer: diagnosis and treatment options. Am Fam Physician. 2004; 69:1133–1140.7. Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004; 22:2395–2403.8. Chau I, Ashley S, Cunningham D. Validation of the Royal Marsden hospital prognostic index in advanced esophagogastric cancer using individual patient data from the REAL 2 study. J Clin Oncol. 2009; 27:e3–e4.9. Koo DH, Ryoo BY, Kim HJ, Ryu MH, Lee SS, Moon JH, et al. A prognostic model in patients who receive chemotherapy for metastatic or recurrent gastric cancer: validation and comparison with previous models. Cancer Chemother Pharmacol. 2011; 68:913–921.10. Takahari D, Boku N, Mizusawa J, Takashima A, Yamada Y, Yoshino T, et al. Determination of prognostic factors in Japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan clinical oncology group 9912. Oncologist. 2014; 19:358–366.11. Catalano V, Graziano F, Santini D, D’Emidio S, Baldelli AM, Rossi D, et al. Second-line chemotherapy for patients with advanced gastric cancer: who may benefit? Br J Cancer. 2008; 99:1402–1407.12. Kanagavel D, Pokataev IA, Fedyanin MY, Tryakin AA, Bazin IS, Narimanov MN, et al. A prognostic model in patients treated for metastatic gastric cancer with second-line chemotherapy. Ann Oncol. 2010; 21:1779–1785.13. Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. American Society of Clinical Oncology. J Clin Oncol. 1996; 14:671–679.14. Sun KY, Xu JB, Chen SL, Yuan YJ, Wu H, Peng JJ, et al. Novel immunological and nutritional-based prognostic index for gastric cancer. World J Gastroenterol. 2015; 21:5961–5971.15. Dorcaratto D, Grande L, Ramón JM, Pera M. Quality of life of patients with cancer of the oesophagus and stomach. Cir Esp. 2011; 89:635–644.16. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014; 383:31–39.17. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–1235.18. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.19. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993; 85:365–376.20. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15:361–387.21. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer;2001.22. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015; 33:550–558.23. Kusano T, Shiraishi N, Shiroshita H, Etoh T, Inomata M, Kitano S. Poor prognosis of advanced gastric cancer with metastatic suprapancreatic lymph nodes. Ann Surg Oncol. 2013; 20:2290–2295.24. Louvet C, Carrat F, Mal F, Mabro M, Beerblock K, Vaillant JC, et al. Prognostic factor analysis in advanced gastric cancer patients treated with hydroxyurea, leucovorin, 5-fluorouracil, and cisplatin (HLFP regimen). Cancer Invest. 2003; 21:14–20.25. Rougier P, Ducreux M, Mahjoubi M, Pignon JP, Bellefqih S, Oliveira J, et al. Efficacy of combined 5-fluorouracil and cisplatinum in advanced gastric carcinomas. A phase II trial with prognostic factor analysis. Eur J Cancer. 1994; 30A:1263–1269.26. Chen SL, Xue N, Wu MT, Chen H, He X, Li JP, et al. Influence of preoperative serum aspartate aminotransferase (AST) level on the prognosis of patients with non-small cell lung cancer. Int J Mol Sci. 2016; 17:1474–1486.27. Zhang K, Lai Y, Axelrod R, Campling B, Hyslop T, Civan J, et al. Modeling the overall survival of patients with advanced-stage non-small cell lung cancer using data of routine laboratory tests. Int J Cancer. 2015; 136:382–391.28. Watine J, Friedberg B. Laboratory variables and stratification of metastatic colorectal cancer patients: recommendations for therapeutic trials and for clinical practice guidelines. Clin Chim Acta. 2004; 345:1–15.29. Watine J, Friedberg B, Bouarioua N. Biological variables and stratification of patients with inoperable non-small-cell bronchial cancer: recommendations for future trials. Cancer Radiother. 2002; 6:209–216.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Ischemic Stroke in a Patient with Metastatic Gastric Cancer after Ramucirumab Chemotherapy
- Anti-vascular endothelial growth factor receptor in colorectal cancer: A review
- Update of Adjuvant Chemotherapy for Resected Gastric Cancer
- Tumor Size as a Prognostic Factor in Gastric Cancer Patient
- Exploratory Analysis of Patients With Gastric/Gastroesophageal Junction Adenocarcinoma With or Without Liver Metastasis From the Phase 3 RAINBOW Study