Immune Netw.
2017 Jun;17(3):192-200. 10.4110/in.2017.17.3.192.
Exacerbation of Japanese Encephalitis by CD11c(hi) Dendritic Cell Ablation Is Associated with an Imbalance in Regulatory Foxp3⺠and IL-17âºCD4⺠Th17 Cells and in Ly-6C(hi) and Ly-6C(lo) Monocytes
- Affiliations
-
- 1College of Veterinary Medicine and Bio-Safety Research Institute, Chonbuk National University, Iksan 54596, Korea. vetvirus@chonbuk.ac.kr
- 2Department of Bioactive Material Sciences, Graduate School, Chonbuk National University, Jeonju 54896, Korea.
- KMID: 2383358
- DOI: http://doi.org/10.4110/in.2017.17.3.192
Abstract
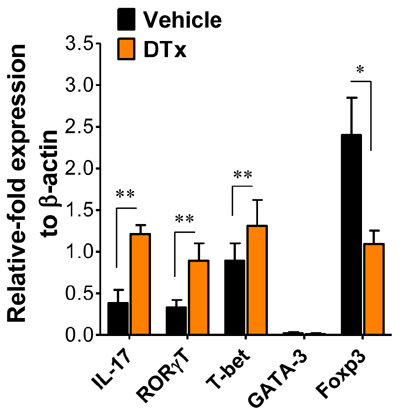
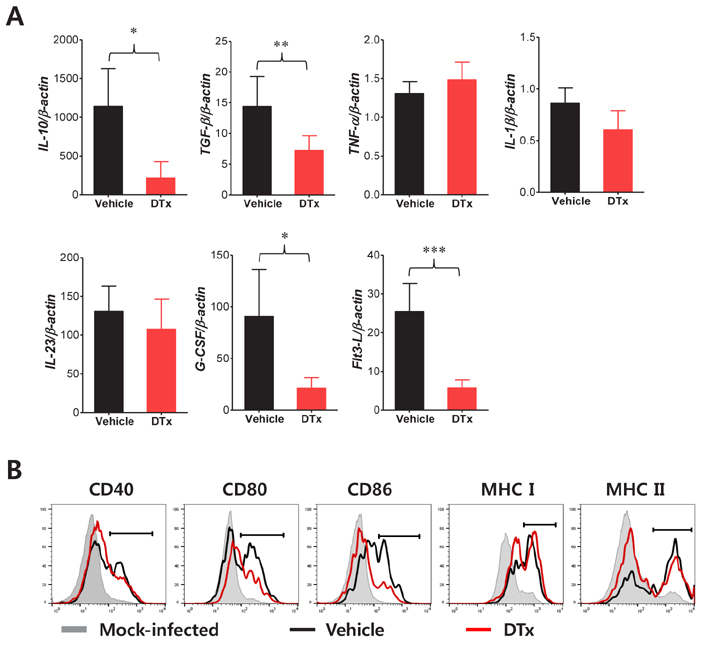
- Japanese encephalitis (JE) is neuroinflammation characterized by uncontrolled infiltration of peripheral leukocytes into the central nervous system (CNS). We previously demonstrated exacerbation of JE following CD11c(hi) dendritic cell (DC) ablation in CD11c-DTR transgenic mice. Moreover, CD11c(hi) DC ablation led to abnormal differentiation of CD11bâºLy-6C(hi) monocytes and enhanced permeability of the blood-brain barrier (BBB), resulting in promoting the progression of JE. Here, we examined changes in lymphoid and myeloid-derived leukocyte subpopulations associated with pro- and anti-inflammation during JE progression. The analyses of this study focused on regulatory CD4âºFoxp3⺠regulatory T cells (Tregs), IL-17âºCD4⺠Th17 cells, and CD11bâºLy-6C(hi) and Ly-6C(lo) monocytes. CD11c(hi) DC ablation resulted in the accumulation of IL-17âºCD4⺠Th17 cells in the CNS, thereby leading to lower ratio of Tregs to Th17 cells. This result was corroborated by the higher expression levels of IL-17 and RORγT in CD4⺠T cells from the brains of CD11c(hi) DC-ablated mice. In addition, CD11c(hi) DC-ablated mice showed higher frequency and total number of inflammatory CD11bâºLy-6C(hi) monocytes, whereas CD11bâºLy-6C(lo) monocytes were detected with lower frequency and total number in CD11c(hi) DC-ablated mice. Furthermore, CD11c(hi) DC ablation altered the phenotype and function of CD11bâºLy-6C(lo) monocytes, resulting in lower levels of activation marker and anti-inflammatory cytokine (IL-10 and TGF-β) expression. Collectively, these results indicate that CD11c(hi) DC ablation caused an imbalance in CD4⺠Th17/Treg cells and CD11bâºLy-6C(hi)/Ly-6C(lo) monocytes in the lymphoid tissue and CNS during JE progression. This imbalanced orchestration of pro- and anti-inflammatory leukocytes following CD11c(hi) DC ablation may contribute to the exacerbation of JE.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Polymeric Nanoparticles Containing Both Antigen and Vitamin D3 Induce Antigen-Specific Immune Suppression
Ho-Hyun Jung, Sang-Hyun Kim, Jun-Hyeok Moon, Seong-Un Jeong, Sundong Jang, Chan-Su Park, Chong-Kil Lee
Immune Netw. 2019;19(3):. doi: 10.4110/in.2019.19.e19.
Reference
-
1. Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, Ginsburg AS. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011; 89:766E–774E.2. Ashhurst TM, Vreden C, Munoz-Erazo L, Niewold P, Watabe K, Terry RL, Deffrasnes C, Getts DR, Cole King NJ. Antiviral macrophage responses in flavivirus encephalitis. Indian J Med Res. 2013; 138:632–647.3. Li F, Wang Y, Yu L, Cao S, Wang K, Yuan J, Wang C, Wang K, Cui M, Fu ZF. Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J Virol. 2015; 89:5602–5614.
Article4. Ye J, Jiang R, Cui M, Zhu B, Sun L, Wang Y, Zohaib A, Dong Q, Ruan X, Song Y, He W, Chen H, Cao S. Etanercept reduces neuroinflammation and lethality in mouse model of Japanese encephalitis. J Infect Dis. 2014; 210:875–889.
Article5. Han YW, Choi JY, Uyangaa E, Kim SB, Kim JH, Kim BS, Kim K, Eo SK. Distinct dictation of Japanese encephalitis virus-induced neuroinflammation and lethality via triggering TLR3 and TLR4 signal pathways. PLoS Pathog. 2014; 10:e1004319.
Article6. Sips GJ, Wilschut J, Smit JM. Neuroinvasive flavivirus infections. Rev Med Virol. 2012; 22:69–87.
Article7. German AC, Myint KS, Mai NT, Pomeroy I, Phu NH, Tzartos J, Winter P, Collett J, Farrar J, Barrett A, Kipar A, Esiri MM, Solomon T. A preliminary neuropathological study of Japanese encephalitis in humans and a mouse model. Trans R Soc Trop Med Hyg. 2006; 100:1135–1145.
Article8. Kim SB, Choi JY, Kim JH, Uyangaa E, Patil AM, Park SY, Lee JH, Kim K, Han YW, Eo SK. Amelioration of Japanese encephalitis by blockage of 4-1BB signaling is coupled to divergent enhancement of type I/II IFN responses and Ly-6C(hi) monocyte differentiation. J Neuroinflammation. 2015; 12:216.
Article9. Aleyas AG, George JA, Han YW, Rahman MM, Kim SJ, Han SB, Kim BS, Kim K, Eo SK. Functional modulation of dendritic cells and macrophages by Japanese encephalitis virus through MyD88 adaptor molecule-dependent and -independent pathways. J Immunol. 2009; 183:2462–2474.
Article10. Terry RL, Getts DR, Deffrasnes C, van Vreden C, Campbell IL, King NJ. Inflammatory monocytes and the pathogenesis of viral encephalitis. J Neuroinflammation. 2012; 9:270.
Article11. Steinman RM, Gutchinov B, Witmer MD, Nussenzweig MC. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983; 157:613–627.
Article12. Kelsall BL, Biron CA, Sharma O, Kaye PM. Dendritic cells at the host-pathogen interface. Nat Immunol. 2002; 3:699–702.
Article13. Cerboni S, Gentili M, Manel N. Diversity of pathogen sensors in dendritic cells. Adv Immunol. 2013; 120:211–237.
Article14. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015; 16:343–353.
Article15. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014; 14:392–404.
Article16. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014; 14:571–578.
Article17. Jung S, Unutmaz D, Wong P, Sano G, De los SK, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002; 17:211–220.
Article18. Kim JH, Choi JY, Kim SB, Uyangaa E, Patil AM, Han YW, Park SY, Lee JH, Kim K, Eo SK. CD11c(hi) dendritic cells regulate Ly-6C(hi) monocyte differentiation to preserve immune-privileged CNS in lethal neuroinflammation. Sci Rep. 2015; 5:17548.
Article19. Kim JH, Hossain FM, Patil AM, Choi JY, Kim SB, Uyangaa E, Park SY, Lee JH, Kim B, Kim K, Eo SK. Ablation of CD11c(hi) dendritic cells exacerbates Japanese encephalitis by regulating blood-brain barrier permeability and altering tight junction/adhesion molecules. Comp Immunol Microbiol Infect Dis. 2016; 48:22–32.
Article20. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014; 5:514.
Article21. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010; 330:841–845.
Article22. Chen BP, Kuziel WA, Lane TE. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. J Immunol. 2001; 167:4585–4592.
Article23. Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000; 192:899–905.
Article24. Campbell DJ. Control of regulatory T cell migration, function, and homeostasis. J Immunol. 2015; 195:2507–2513.
Article25. Ding Y, Xu J, Bromberg JS. Regulatory T cell migration during an immune response. Trends Immunol. 2012; 33:174–180.
Article26. Kim JH, Patil AM, Choi JY, Kim SB, Uyangaa E, Hossain FM, Park SY, Lee JH, Eo SK. CCR5 ameliorates Japanese encephalitis via dictating the equilibrium of regulatory CD4(+)Foxp3(+) T and IL-17(+)CD4(+) Th17 cells. J Neuroinflammation. 2016; 13:223.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of macrophages in liver cirrhosis: fibrogenesis and resolution
- The Expression of Cytokine and Chemokine Related with Dendritic Cell and Effector T Cell in Psoriatic Lesions
- IL-17A-Producing Foxp3⺠Regulatory T Cells and Human Diseases
- IL-17A and Th17 Cells Contribute to Endometrial Cell Survival by Inhibiting Apoptosis and NK Cell Mediated Cytotoxicity of Endometrial Cells via ERK1/2 Pathway
- A Study of the Balance of Th17 Cells and Regulatory T Cells in Psoriasis