Ann Dermatol.
2016 Oct;28(5):579-585. 10.5021/ad.2016.28.5.579.
Induction of Interleukin-22 (IL-22) production in CD4+ T Cells by IL-17A Secreted from CpG-Stimulated Keratinocytes
- Affiliations
-
- 1Department of Dermatology, Chungnam National University School of Medicine, Daejeon, Korea. jhoon@cnu.ac.kr
- KMID: 2382882
- DOI: http://doi.org/10.5021/ad.2016.28.5.579
Abstract
- BACKGROUND
Interleukin-17A (IL-17A) is mainly secreted from Th17 cells that are activated by various stimuli including CpG oligodeoxynucleotide, a Toll-like receptor 9 (TLR9) ligand. Recently, it has been demonstrated that keratinocytes play an important role in the pathogenesis of psoriasis.
OBJECTIVE
To investigate the potential role of keratinocytes, we examined whether TLR9 ligand CpG induces IL-17A expression in keratinocytes.
METHODS
We used HaCaT keratinocytes as a model system, and determined CpG-induced IL-17A using enzyme-linked immunosorbent assay and Western blot.
RESULTS
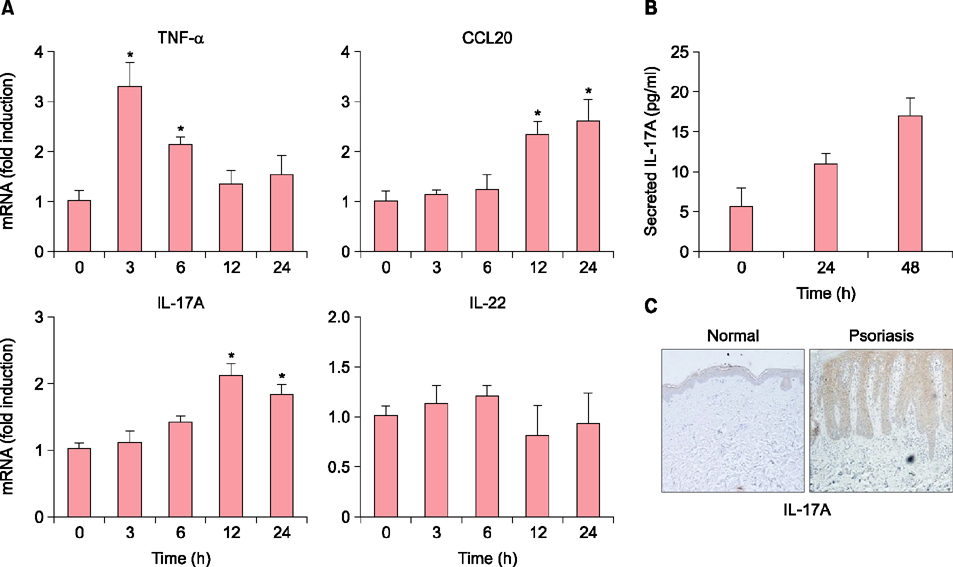
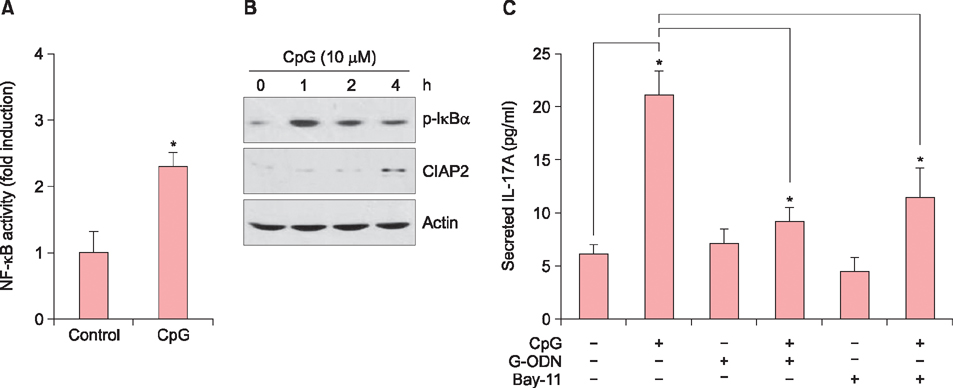
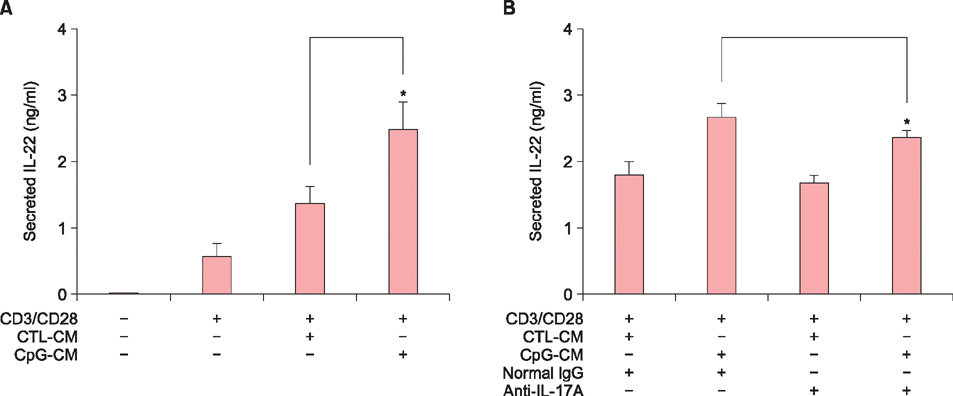
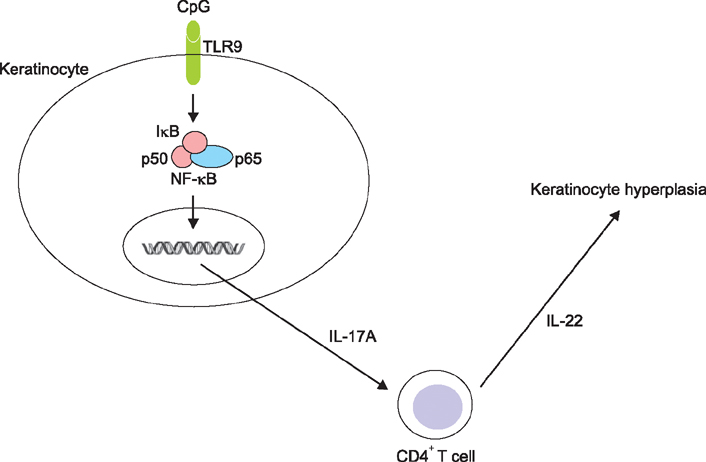
When HaCaT keratinocytes were treated with CpG, the expression of several cytokines including IL-17A, tumor necrosis factor-α and CCL20 was markedly increased. Treatment with nuclear factor (NF)-κB inhibitor significantly blocked the CpG-induced IL-17A production, indicating that CpG induced IL-17A expression through the NF-κB signaling pathway. In addition, IL-17A secreted from keratinocytes stimulated the CD4⺠T cells, resulting in strong induction of IL-22 production.
CONCLUSION
Since IL-22 is an important mediator for psoriatic inflammation, our data suggest that keratinocytes can participate in the pathogenesis of psoriasis via the TLR9-dependent IL-17A production.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Inhibition of Poly(I:C)-Induced Inflammation by Salvianolic Acid A in Skin Keratinocytes
Qing-Ling Zhang, Ri-Hua Jiang, Xue Mei Li, Jung-Woo Ko, Chang Deok Kim, Ming Ji Zhu, Jeung-Hoon Lee
Ann Dermatol. 2019;31(3):279-285. doi: 10.5021/ad.2019.31.3.279.
Reference
-
1. Li Y, Begovich AB. Unraveling the genetics of complex diseases: susceptibility genes for rheumatoid arthritis and psoriasis. Semin Immunol. 2009; 21:318–327.
Article2. Nakajima H, Nakajima K, Tarutani M, Morishige R, Sano S. Kinetics of circulating Th17 cytokines and adipokines in psoriasis patients. Arch Dermatol Res. 2011; 303:451–455.
Article3. Becher B, Pantelyushin S. Hiding under the skin: interleukin-17-producing γδ T cells go under the skin? Nat Med. 2012; 18:1748–1750.
Article4. Pappu R, Rutz S, Ouyang W. Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol. 2012; 33:343–349.
Article5. Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008; 226:57–79.
Article6. Truchetet ME, Mossalayi MD, Boniface K. IL-17 in the rheumatologist's line of sight. Biomed Res Int. 2013; DOI: 10.1155/2013/295132.
Article7. Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011; 34:149–162.
Article8. Zhang L, Yang XQ, Cheng J, Hui RS, Gao TW. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol. 2010; 135:108–117.
Article9. Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013; 168:402–411.
Article10. Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007; 7:179–190.
Article11. Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001; 1:135–145.
Article12. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004; 5:987–995.
Article13. McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005; 125:1–8.
Article14. Kawai K. Expression of functional toll-like receptors on cultured human epidermal keratinocytes. J Invest Dermatol. 2003; 121:217.
Article15. Miller LS, Sørensen OE, Liu PT, Jalian HR, Eshtiaghpour D, Behmanesh BE, et al. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J Immunol. 2005; 174:6137–6143.
Article16. Li ZJ, Sohn KC, Choi DK, Shi G, Hong D, Lee HE, et al. Roles of TLR7 in activation of NF-κB signaling of keratinocytes by imiquimod. PLoS One. 2013; 8:e77159.
Article17. Köllisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, et al. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005; 114:531–541.
Article18. Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007; 127:331–341.
Article19. Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One. 2010; 5:e14108.
Article20. Garber K. Genetics: deep exploration. Nature. 2012; 492:S56–S57.
Article21. Balato A, Lembo S, Mattii M, Schiattarella M, Marino R, De Paulis A, et al. IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp Dermatol. 2012; 21:892–894.
Article22. Gutowska-Owsiak D, Ogg GS. The epidermis as an adjuvant. J Invest Dermatol. 2012; 132:940–948.
Article23. Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007; 29:15–26.
Article24. Terhorst D, Kalali BN, Ollert M, Ring J, Mempel M. The role of toll-like receptors in host defenses and their relevance to dermatologic diseases. Am J Clin Dermatol. 2010; 11:1–10.
Article25. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009; 22:240–273.
Article26. Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011; 12:695–708.
Article27. Jeong MS, Kim JY, Lee HI, Seo SJ. Calcitriol may down-regulate mRNA over-expression of toll-like receptor-2 and -4, LL-37 and proinflammatory cytokines in cultured human keratinocytes. Ann Dermatol. 2014; 26:296–302.
Article28. Venugopal PG, Nutman TB, Semnani RT. Activation and regulation of toll-like receptors (TLRs) by helminth parasites. Immunol Res. 2009; 43:252–263.
Article29. Schauber J, Dombrowski Y, Besch R. Pathogenic DNA: cytosolic DNA promotes inflammation in psoriasis. Cell Cycle. 2011; 10:3038–3039.
Article30. Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005; 64:Suppl 2. ii30–ii36.
Article31. Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007; 150:407–415.
Article32. Dyring-Andersen B, Skov L, Løvendorf MB, Bzorek M, Søndergaard K, Lauritsen JP, et al. CD4(+) T cells producing interleukin (IL)-17, IL-22 and interferon-γ are major effector T cells in nickel allergy. Contact Dermatitis. 2013; 68:339–347.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Interleukin 4 on the Production of Interleukin 6 in Human Keratinocytes
- I-Kappa-B-Zeta Regulates Interleukin-17A/ Tumor Necrosis Factor-Alpha Mediated Synergistic Induction of Interleukin-19 and Interleukin-20 in Humane Keratinocytes
- Effects of Thyroid Hormone on Preduction of Interleukin-6 and Interleukin-11 in Human Bone Marrow Stromal Cells
- Interleukin-17 and Interleukin-22 Induced Proinflammatory Cytokine Production in Keratinocytes via Inhibitor of Nuclear Factor kappaB Kinase-alpha Expression
- The effect of IL-1 from keratinocytes on production of IL-2 by peripheral lymphocytes