Int J Stem Cells.
2015 May;8(1):90-98. 10.15283/ijsc.2015.8.1.90.
Attachment and Differentiation of Human Umbilical Cord Stem Cells on to the Tooth Root Surface with and without the Use of Fibroblast Growth Factor-An In Vitro Study
- Affiliations
-
- 1Krishnadevaraya College of Dental Sciences & Hospital, Hunasamaranahalli, (via) Yelahanka, Krishnadevarayanagar, Bangalore (North), India. drjoannpaulinegeorge@gmail.com
- 2Sri Raghavendra Biotechnologies Pvt. Ltd. Bangalore, India.
- KMID: 2380802
- DOI: http://doi.org/10.15283/ijsc.2015.8.1.90
Abstract
- BACKGROUND AND OBJECTIVES
The purpose of this first of its kind study was to analyse the growth, development and attachment of cultured human umbilical cord stem cells alone or supplemented with basic Fibroblast Growth Factor (bFGF) on both healthy and periodontally diseased tooth surfaces in vitro.
METHODS
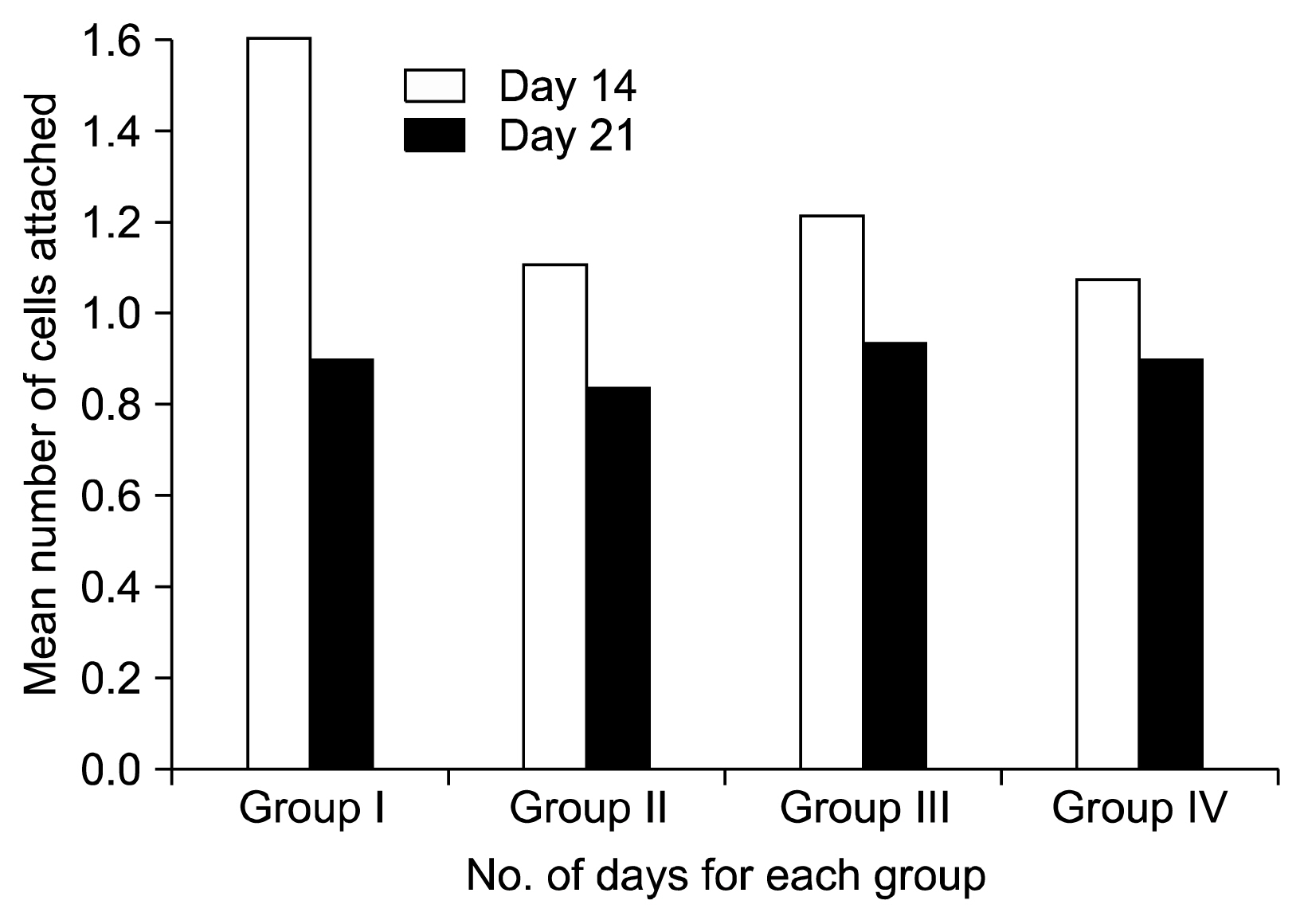
Four groups of 12 root surface scaffolds each were classified as Group I- healthy root surfaces; Group II- periodontally diseased; Group III- Healthy with bFGF and Group IV- periodontally diseased root with bFGF. bFGF was applied in the concentration of 8 ng/ml on to the surface followed by incubation of cultured human umbilical cord stem cells (hUCMSCs) on the scaffolds. Scanning electron microscopy observations were made on 14th and 21st days to assess the proliferation and morphology of cells attached on the tooth surface.
RESULTS
Cultured hUCMSCs demonstrated adhesion to tooth root scaffold. All the groups showed a significant increase in the number of cell attachment from 14th day to 21st day. The groups with bFGF showed a significant increase in attachment of cells when compared to the groups without bFGF. The cells showed an increase in number of flat cells from 14th day to 21st day in all the groups indicating an increased maturity of cells. Periodontally diseased groups had less maturity of cells than healthy groups. The groups supplemented with bFGF, had more mature cells than the groups without bFGF.
CONCLUSIONS
hUCMSCs have the propensity to differentiate into cells that have the capacity to bind to root surfaces. hUCMSCs incubated with bFGF showed better proliferation and attachment to tooth root surfaces. The role of hUCMSCs can be further explored for periodontal regeneration.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Mudda JA, Bajaj M. Stem cell therapy: a challenge to periodontist. Indian J Dent Res. 2011; 22:132–139. DOI: 10.4103/0970-9290.79978. PMID: 21525691.
Article2. Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005; 8:191–199. DOI: 10.1111/j.1601-6343.2005.00331.x. PMID: 16022721.
Article3. Chen FM, Sun HH, Lu H, Yu Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials. 2012; 33:6320–6344. DOI: 10.1016/j.biomaterials.2012.05.048. PMID: 22695066.
Article4. Taghizadeh RR, Cetrulo KJ, Cetrulo CL. Wharton’s Jelly stem cells: future clinical applications. Placenta. 2011; 32( Suppl 4):S311–S315. DOI: 10.1016/j.placenta.2011.06.010.
Article5. Kitamura M, Akamatsu M, Machigashira M, Hara Y, Sakagami R, Hirofuji T, Hamachi T, Maeda K, Yokota M, Kido J, Nagata T, Kurihara H, Takashiba S, Sibutani T, Fukuda M, Noguchi T, Yamazaki K, Yoshie H, Ioroi K, Arai T, Nakagawa T, Ito K, Oda S, Izumi Y, Ogata Y, Yamada S, Shimauchi H, Kunimatsu K, Kawanami M, Fujii T, Furuichi Y, Furuuchi T, Sasano T, Imai E, Omae M, Yamada S, Watanuki M, Murakami S. FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial. J Dent Res. 2011; 90:35–40. DOI: 10.1177/0022034510384616.6. Belal MH, Watanabe H, Ichinose S, Ishikawa I. A time-dependent effect of PDGF-BB on adhesion and growth of cultured fibroblasts to root surfaces. Oral Dis. 2006; 12:543–552. DOI: 10.1111/j.1601-0825.2006.01233.x. PMID: 17054766.
Article7. Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010; 62:2467–2475. DOI: 10.1002/art.27548. PMID: 20506343.
Article8. Inanç B, Elçin AE, Elçin YM. In vitro differentiation and attachment of human embryonic stem cells on periodontal tooth root surfaces. Tissue Eng Part A. 2009; 15:3427–3435. DOI: 10.1089/ten.tea.2008.0380. PMID: 19405785.
Article9. Gamal AY, Mailhot JM, Garnick JJ, Newhouse R, Sharawy MM. Human periodontal ligament fibroblast response to PDGF-BB and IGF-1 application on tetracycline HCI conditioned root surfaces. J Clin Periodontol. 1998; 25:404–412. DOI: 10.1111/j.1600-051X.1998.tb02463.x. PMID: 9650878.
Article10. Ramasamy R, Tong CK, Yip WK, Vellasamy S, Tan BC, Seow HF. Basic fibroblast growth factor modulates cell cycle of human umbilical cord-derived mesenchymal stem cells. Cell Prolif. 2012; 45:132–139. DOI: 10.1111/j.1365-2184.2012.00808.x. PMID: 22309282.
Article11. Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006; 24:462–471. DOI: 10.1634/stemcells.2004-0331.
Article12. Neubauer M, Hacker M, Bauer-Kreisel P, Weiser B, Fischbach C, Schulz MB, Goepferich A, Blunk T. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng. 2005; 11:1840–1851.
Article13. Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004; 86-A:1541–1558. PMID: 15252108.
Article14. Li TX, Yuan J, Chen Y, Pan LJ, Song C, Bi LJ, Jiao XH. Differentiation of mesenchymal stem cells from human umbilical cord tissue into odontoblast-like cells using the conditioned medium of tooth germ cells in vitro. Biomed Res Int. 2013; 2013:218543. PMID: 23762828. PMCID: 3666309.15. Bluteau G, Luder HU, De Bari C, Mitsiadis TA. Stem cells for tooth engineering. Eur Cell Mater. 2008; 16:1–9. PMID: 18671204.
Article16. Hagmann S, Moradi B, Frank S, Dreher T, Kämmerer PW, Richter W, Gotterbarm T. FGF-2 addition during expansion of human bone marrow-derived stromal cells alters MSC surface marker distribution and chondrogenic differentiation potential. Cell Prolif. 2013; 46:396–407. PMID: 23869761.
Article17. Murakami S. Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol 2000. 2011; 56:188–208. PMID: 21501244.
Article18. Feist IS, De Micheli G, Carneiro SR, Eduardo CP, Miyagi S, Marques MM. Adhesion and growth of cultured human gingival fibroblasts on periodontally involved root surfaces treated by Er:YAG laser. J Periodontol. 2003; 74:1368–1375. PMID: 14584872.
Article19. Hakki SS, Korkusuz P, Berk G, Dundar N, Saglam M, Bozkurt B, Purali N. Comparison of Er, Cr:YSGG laser and hand instrumentation on the attachment of periodontal ligament fibroblasts to periodontally diseased root surfaces: an in vitro study. J Periodontol. 2010; 81:1216–1225. PMID: 20476883.
Article20. Morito A, Kida Y, Suzuki K, Inoue K, Kuroda N, Gomi K, Arai T, Sato T. Effects of basic fibroblast growth factor on the development of the stem cell properties of human dental pulp cells. Arch Histol Cytol. 2009; 72:51–64. PMID: 19789412.
Article21. Wu J, Huang GT, He W, Wang P, Tong Z, Jia Q, Dong L, Niu Z, Ni L. Basic fibroblast growth factor enhances stemness of human stem cells from the apical papilla. J Endod. 2012; 38:614–622. DOI: 10.1016/j.joen.2012.01.014. PMID: 22515889. PMCID: 3499972.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Hepatocyte Growth Factor is the Key Cytokine in Stimulating Potential Stem Cells in the Cord Blood into Hepatic Lineage Cells
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- In Vitro Culture of Mast Cells from Human Umbilical Cord Blood Cells
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells