Clin Exp Otorhinolaryngol.
2017 Jun;10(2):137-142. 10.21053/ceo.2016.00444.
Functional Significance of Medial Olivocochlear System Morphology in the Mouse Cochlea
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea. snparkmd@catholic.ac.kr
- KMID: 2380430
- DOI: http://doi.org/10.21053/ceo.2016.00444
Abstract
OBJECTIVES
Baso-apical gradients exist in various cochlear structures including medial olivocochlear (MOC) efferent system. This study investigated the cochlear regional differentials in the function and morphology of the MOC system, and addressed the functional implications of regional MOC efferent terminals (ETs) in the mouse cochlea.
METHODS
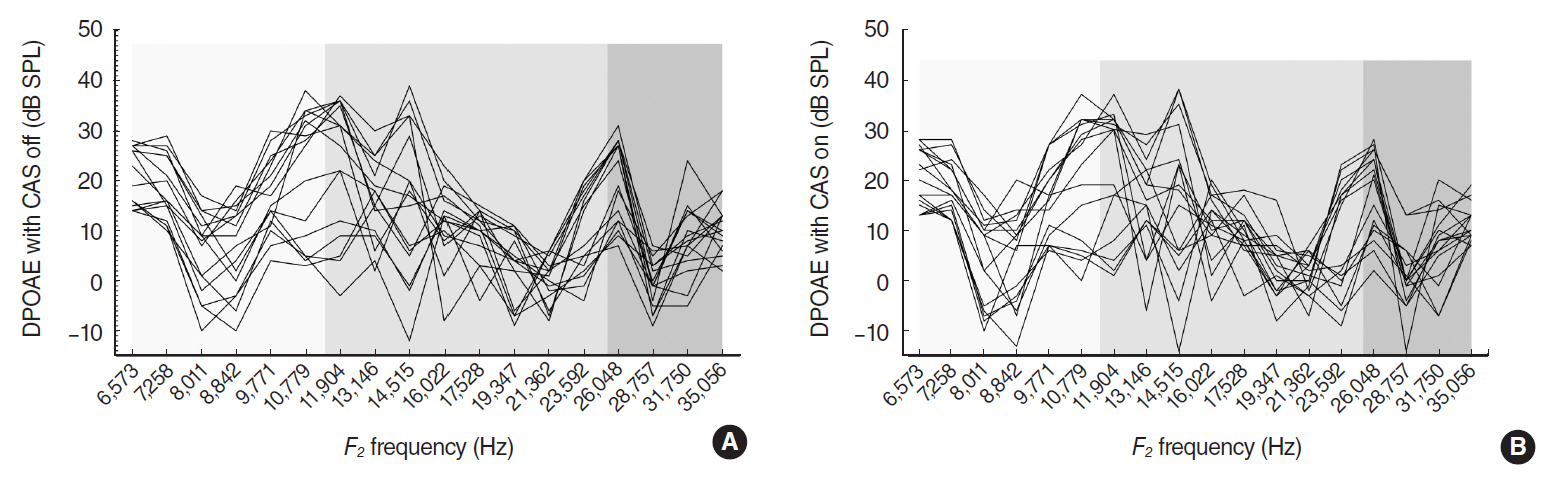
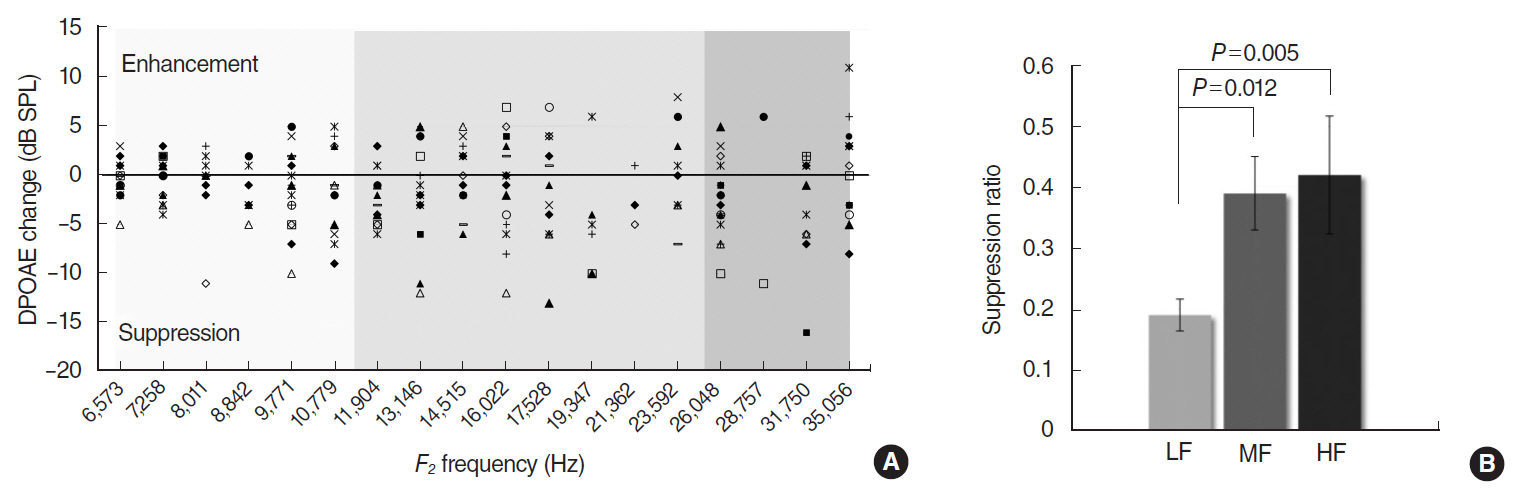
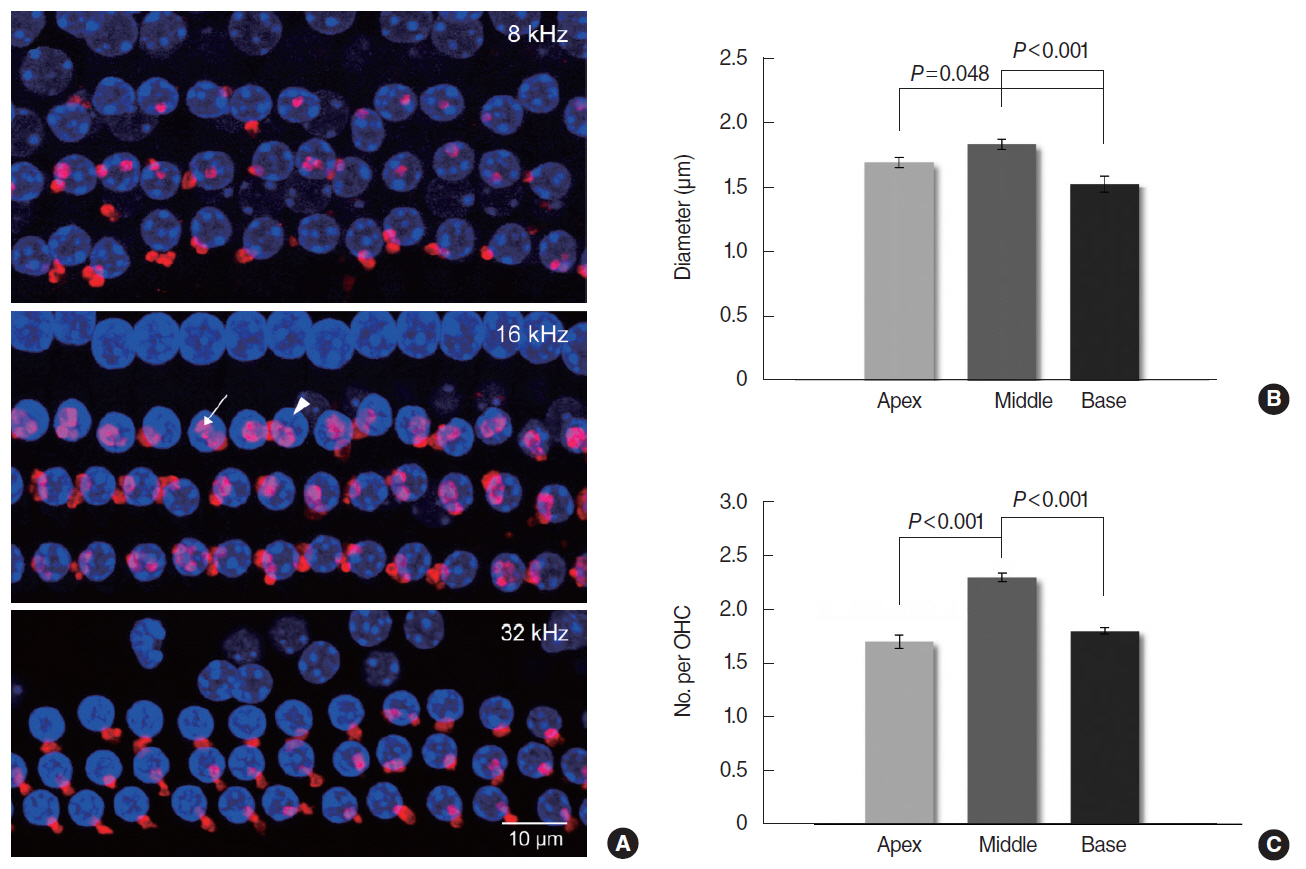
In CBA/J mice, MOC reflex (MOCR) was assessed based on the distortion product otoacoustic emission in the absence and presence of contralateral acoustic stimulation. High, middle, and low frequencies were grouped according to a mouse place-frequency map. Cochlear whole mounts were immunostained for ETs with anti-α-synuclein and examined using confocal laser scanning microscopy. The diameters of ETs and the number of ETs per outer hair cell were measured from the z-stack images of the basal, middle and apical regions, respectively.
RESULTS
The middle cochlear region expressed large, clustered MOC ETs with strong MOCR, the base expressed small, less clustered ETs with strong MOCR, and the apex expressed large, but less clustered ETs with weak MOCR.
CONCLUSION
The mouse cochlea demonstrated regional differentials in the function and morphology of the MOC system. Strong MOCR along with superior MOC morphology in the middle region may contribute to "˜signal detection in noise,' the primary efferent function, in the best hearing frequencies. Strong MOCR in spite of inferior MOC morphology in the base may reflect the importance of "˜protection from noise trauma' in the high frequencies.
Keyword
Figure
Reference
-
1. Christopher Kirk E, Smith DW. Protection from acoustic trauma is not a primary function of the medial olivocochlear efferent system. J Assoc Res Otolaryngol. 2003; Dec. 4(4):445–65.
Article2. Rabbitt RD, Brownell WE. Efferent modulation of hair cell function. Curr Opin Otolaryngol Head Neck Surg. 2011; Oct. 19(5):376–81.
Article3. Smith DW, Keil A. The biological role of the medial olivocochlear efferents in hearing: separating evolved function from exaptation. Front Syst Neurosci. 2015; Feb. 9:12.
Article4. Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci. 2000; Jun. 20(12):4701–7.
Article5. Brown MC, de Venecia RK, Guinan JJ Jr. Responses of medial olivocochlear neurons: specifying the central pathways of the medial olivocochlear reflex. Exp Brain Res. 2003; Dec. 153(4):491–8.6. Guinan JJ Jr. Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006; Dec. 27(6):589–607.
Article7. Mann ZF, Kelley MW. Development of tonotopy in the auditory periphery. Hear Res. 2011; Jun. 276(1-2):2–15.
Article8. Vater M, Kossl M. Comparative aspects of cochlear functional organization in mammals. Hear Res. 2011; Mar. 273(1-2):89–99.
Article9. Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006; Feb. 27(1):1–19.
Article10. Guinan JJ Jr. Cochlear efferent innervation and function. Curr Opin Otolaryngol Head Neck Surg. 2010; Oct. 18(5):447–53.
Article11. Maison SF, Adams JC, Liberman MC. Olivocochlear innervation in the mouse: immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization. J Comp Neurol. 2003; Jan. 455(3):406–16.
Article12. Maison SF, Rosahl TW, Homanics GE, Liberman MC. Functional role of GABAergic innervation of the cochlea: phenotypic analysis of mice lacking GABA(A) receptor subunits alpha 1, alpha 2, alpha 5, alpha 6, beta 2, beta 3, or delta. J Neurosci. 2006; Oct. 26(40):103.13. Zhu X, Vasilyeva ON, Kim S, Jacobson M, Romney J, Waterman MS, et al. Auditory efferent feedback system deficits precede age-related hearing loss: contralateral suppression of otoacoustic emissions in mice. J Comp Neurol. 2007; Aug. 503(5):593–604.
Article14. Viberg A, Canlon B. The guide to plotting a cochleogram. Hear Res. 2004; Nov. 197(1-2):1–10.
Article15. Zhang F, Boettcher FA, Sun XM. Contralateral suppression of distortion product otoacoustic emissions: effect of the primary frequency in Dpgrams. Int J Audiol. 2007; Apr. 46(4):187–95.
Article16. Wagner W, Heppelmann G, Muller J, Janssen T, Zenner HP. Olivocochlear reflex effect on human distortion product otoacoustic emissions is largest at frequencies with distinct fine structure dips. Hear Res. 2007; Jan. 223(1-2):83–92.
Article17. Shaffer LA, Withnell RH, Dhar S, Lilly DJ, Goodman SS, Harmon KM. Sources and mechanisms of DPOAE generation: implications for the prediction of auditory sensitivity. Ear Hear. 2003; Oct. 24(5):367–79.
Article18. Shera CA. Mechanisms of mammalian otoacoustic emission and their implications for the clinical utility of otoacoustic emissions. Ear Hear. 2004; Apr. 25(2):86–97.
Article19. Mishra SK, Abdala C. Stability of the medial olivocochlear reflex as measured by distortion product otoacoustic emissions. J Speech Lang Hear Res. 2015; Feb. 58(1):122–34.
Article20. Willott JF. Overview of methods for assessing the mouse auditory system. Curr Protoc Neurosci. 2006; Feb. Chapter 8:Unit8.21A. http://dx.doi.org/10.1002/0471142301.ns0821as34.
Article21. Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005; Apr. 202(1-2):63–73.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Vestibular Organ and Cochlea
- Distribution of Calcitonin Gene-Related Peptide Immunoreactive Nerve Fibers in Rat Cochlea and Brainstem Auditory Nuclei
- The Effect of Contralateral Acoustic Stimulation on the Latency of Distortion Product Otoacoustic Emissions
- Comparison of Cochlear Morphology and Apoptosis in Mouse Models of Presbycusis
- A Case of Cholesteatoma Extended Both into the Cochlea and IAC