Cancer Res Treat.
2015 Jan;47(1):101-109. 10.4143/crt.2013.192.
TGF-beta Suppresses COX-2 Expression by Tristetraprolin-Mediated RNA Destabilization in A549 Human Lung Cancer Cells
- Affiliations
-
- 1Cancer Research Institute, Seoul National University, Seoul, Korea. moisa@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Translational Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2380395
- DOI: http://doi.org/10.4143/crt.2013.192
Abstract
- PURPOSE
Overexpression of cyclooxygenase 2 (COX-2) is thought to promote survival of transformed cells. Transforming growth factor beta (TGF-beta) exerts anti-proliferative effects on a broad range of epithelial cells. In the current study, we investigated whether TGF-beta can regulate COX-2 expression in A549 human lung adenocarcinoma cells, which are TGF-beta-responsive and overexpress COX-2.
MATERIALS AND METHODS
Western blotting, Northern blotting, and mRNA stability assays were performed to demonstrate that COX-2 protein and mRNA expression were suppressed by TGF-beta. We also evaluated the effects of tristetraprolin (TTP) on COX-2 mRNA using RNA interference.
RESULTS
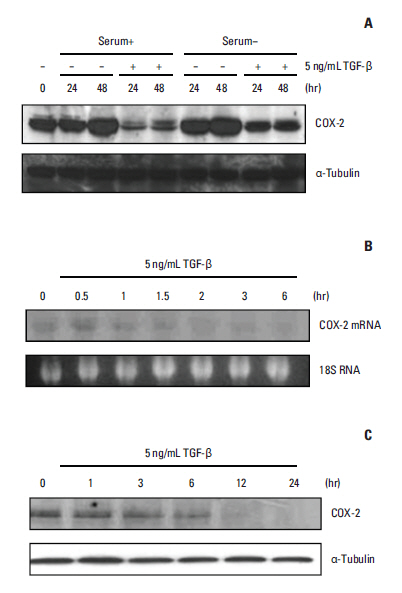
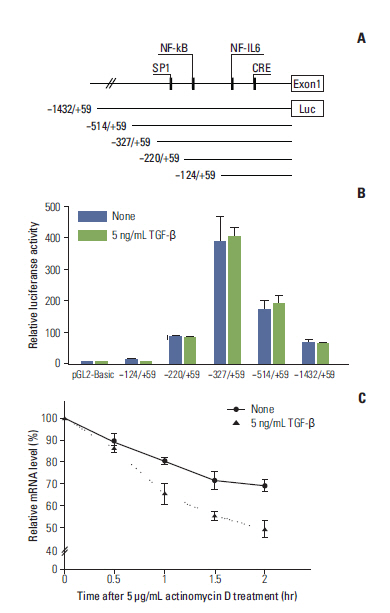
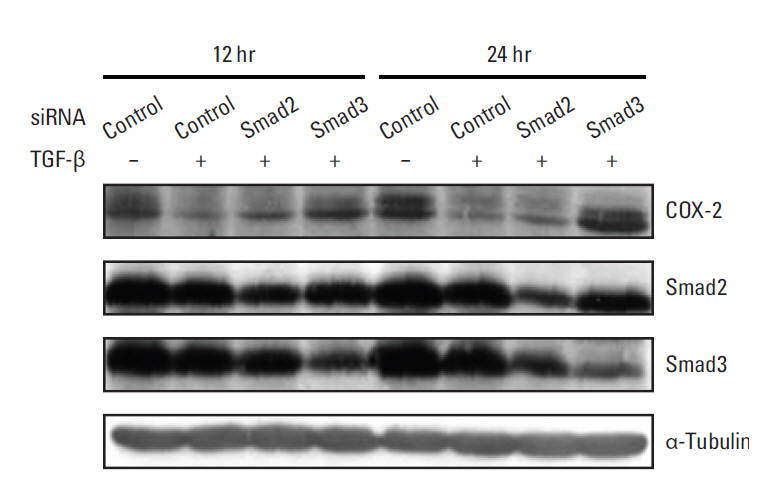
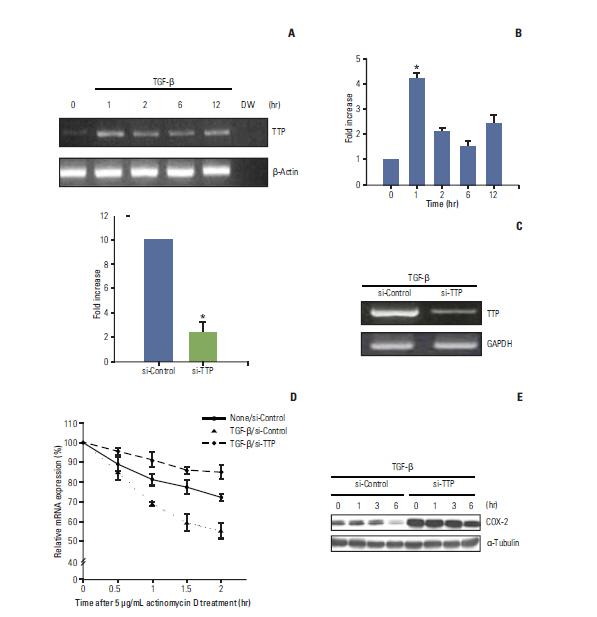
We demonstrated that COX-2 mRNA and protein expression were both significantly suppressed by TGF-beta. An actinomycin D chase experiment demonstrated that COX-2 mRNA was more rapidly degraded in the presence of TGF-beta, suggesting that TGF-beta-induced inhibition of COX-2 expression is achieved via decreased mRNA stability. We also found that TGF-beta rapidly and transiently induced the expression of TTP, a well-known mRNA destabilizing factor, before suppression of COX-2 mRNA expression was observed. Using RNA interference, we confirmed that increased TTP levels play a pivotal role in the destabilization of COX-2 mRNA by TGF-beta. Furthermore, we showed that Smad3 is essential to TTP-dependent down-regulation of COX-2 expression in response to TGF-beta.
CONCLUSION
The results of this study show that TGF-beta down-regulated COX-2 expression via mRNA destabilization mediated by Smad3/TTP in A549 cells.
MeSH Terms
-
Adenocarcinoma
Blotting, Northern
Blotting, Western
Cyclooxygenase 2
Dactinomycin
Down-Regulation
Epithelial Cells
Humans
Lung
Lung Neoplasms*
RNA Interference
RNA Stability
RNA*
RNA, Messenger
Transforming Growth Factor beta*
Tristetraprolin
Cyclooxygenase 2
Dactinomycin
RNA
RNA, Messenger
Transforming Growth Factor beta
Tristetraprolin
Figure
Cited by 1 articles
-
Antitumor Effect of KX-01 through Inhibiting Src Family Kinases and Mitosis
Seongyeong Kim, Ahrum Min, Kyung-Hun Lee, Yaewon Yang, Tae-Yong Kim, Jee Min Lim, So Jung Park, Hyun-Jin Nam, Jung Eun Kim, Sang-Hyun Song, Sae-Won Han, Do-Youn Oh, Jee Hyun Kim, Tae-You Kim, David Hangauer, Johnson Yiu-Nam Lau, Kyongok Im, Dong Soon Lee, Yung-Jue Bang, Seock-Ah Im
Cancer Res Treat. 2017;49(3):643-655. doi: 10.4143/crt.2016.168.
Reference
-
References
1. Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001; 1:11–21.
Article2. Setia S, Vaish V, Sanyal SN. Chemopreventive effects of NSAIDs as inhibitors of cyclooxygenase-2 and inducers of apoptosis in experimental lung carcinogenesis. Mol Cell Biochem. 2012; 366:89–99.
Article3. Vo BT, Morton D Jr, Komaragiri S, Millena AC, Leath C, Khan SA. TGF-beta effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology. 2013; 154:1768–79.4. Tian M, Schiemann WP. PGE2 receptor EP2 mediates the antagonistic effect of COX-2 on TGF-beta signaling during mammary tumorigenesis. FASEB J. 2010; 24:1105–16.5. Heasley LE, Thaler S, Nicks M, Price B, Skorecki K, Nemenoff RA. Induction of cytosolic phospholipase A2 by oncogenic Ras in human non-small cell lung cancer. J Biol Chem. 1997; 272:14501–4.
Article6. Zhou F, Gao G, Ren S, Li X, He Y, Zhou C. The association between COX-2 polymorphisms and hematologic toxicity in patients with advanced non-small-cell lung cancer treated with platinum-based chemotherapy. PLoS One. 2013; 8:e61585.
Article7. Ruegg C, Zaric J, Stupp R. Non steroidal anti-inflammatory drugs and COX-2 inhibitors as anti-cancer therapeutics: hypes, hopes and reality. Ann Med. 2003; 35:476–87.8. Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998; 12:1063–73.
Article9. Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000; 103:295–309.10. Kim CH, Park SY, Yoo J. Expression of transforming growth factor beta1 and E-cadherin proteins in pulmonary adenocarcinoma: its significance in tumor progression. Cancer Res Treat. 2013; 45:118–25.
Article11. Neil JR, Johnson KM, Nemenoff RA, Schiemann WP. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis. 2008; 29:2227–35.12. Chen CY, Gherzi R, Ong SE, Chan EL, Raijmakers R, Pruijn GJ, et al. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001; 107:451–64.
Article13. Sanduja S, Blanco FF, Young LE, Kaza V, Dixon DA. The role of tristetraprolin in cancer and inflammation. Front Biosci (Landmark Ed). 2012; 17:174–88.
Article14. Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998; 281:1001–5.15. Sawaoka H, Dixon DA, Oates JA, Boutaud O. Tristetraprolin binds to the 3'-untranslated region of cyclooxygenase-2 mRNA. A polyadenylation variant in a cancer cell line lacks the binding site. J Biol Chem. 2003; 278:13928–35.16. Sheng H, Shao J, Dixon DA, Williams CS, Prescott SM, DuBois RN, et al. Transforming growth factor-beta1 enhances Ha-rasinduced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. J Biol Chem. 2000; 275:6628–35.17. Park YG, Kang SK, Kim WJ, Lee YC, Kim CH. Effects of TGFbeta, TNF-alpha, IL-beta and IL-6 alone or in combination, and tyrosine kinase inhibitor on cyclooxygenase expression, prostaglandin E2 production and bone resorption in mouse calvarial bone cells. Int J Biochem Cell Biol. 2004; 36:2270–80.18. Li W, Yue W, Zhang L, Zhao X, Ma L, Yang X, et al. COX-2 silencing inhibits cell proliferation in A549 cell. Chin-Ger J Clin Oncol. 2011; 10:423–7.
Article19. Ogawa K, Chen F, Kim YJ, Chen Y. Transcriptional regulation of tristetraprolin by transforming growth factor-beta in human T cells. J Biol Chem. 2003; 278:30373–81.20. Ungefroren H, Groth S, Sebens S, Lehnert H, Gieseler F, Fandrich F. Differential roles of Smad2 and Smad3 in the regulation of TGF-beta1-mediated growth inhibition and cell migration in pancreatic ductal adenocarcinoma cells: control by Rac1. Mol Cancer. 2011; 10:67.21. Cha HJ, Lee HH, Chae SW, Cho WJ, Kim YM, Choi HJ, et al. Tristetraprolin downregulates the expression of both VEGF and COX-2 in human colon cancer. Hepatogastroenterology. 2011; 58:790–5.22. Tian M, Schiemann WP. The TGF-beta paradox in human cancer: an update. Future Oncol. 2009; 5:259–71.23. Kong F, Jirtle RL, Huang DH, Clough RW, Anscher MS. Plasma transforming growth factor-beta1 level before radiotherapy correlates with long term outcome of patients with lung carcinoma. Cancer. 1999; 86:1712–9.24. Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGFbeta targeted cancer therapy. Int J Biol Sci. 2012; 8:964–78.25. Comerci JT Jr, Runowicz CD, Fields AL, Romney SL, Palan PR, Kadish AS, et al. Induction of transforming growth factor beta-1 in cervical intraepithelial neoplasia in vivo after treatment with beta-carotene. Clin Cancer Res. 1997; 3:157–60.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Regulation of FOXP3 Expression by the Treatment of TGF-beta and the Modification of DNA Methylation in Lung Cancer Cell Lines
- Relation between Cyclooxygenase-2 and Polo-like Kinase-1 in Non-Small Cell Lung Cancer
- beta-Lapachone suppresses radiation-induced activation of nuclear factor-kappaB
- Down-regulation of IL-1beta-induced COX-2 Expression in A549 Lung Cancer Cells at Transcriptional Level by Leptomycin B Involves Inhibition of the IkappaB-alpha/NF-kappaB Pathway but Independent of CRM1
- Cardamonin Suppresses TGF-beta1-Induced Epithelial Mesenchymal Transition via Restoring Protein Phosphatase 2A Expression