Cancer Res Treat.
2015 Jan;47(1):55-63. 10.4143/crt.2013.165.
Ovarian Ablation Using Goserelin Improves Survival of Premenopausal Patients with Stage II/III Hormone Receptor-Positive Breast Cancer without Chemotherapy-Induced Amenorrhea
- Affiliations
-
- 1Departments of Obstetrics and Gynecology, The First Affiliated Hospital of Xiamen University, Xiamen, China.
- 2Departments of Radiation Oncology, The First Affiliated Hospital of Xiamen University, Xiamen, China.
- 3Department of Medical Oncology, The Central Hospital of Xinxiang, Xinxiang, China.
- 4Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China. hezhy@sysucc.org.cn
- KMID: 2380390
- DOI: http://doi.org/10.4143/crt.2013.165
Abstract
- PURPOSE
The purpose of this study was to assess the value of ovarian ablation using goserelin in premenopausal patients with stage II/III hormone receptor-positive breast cancer without chemotherapy-induced amenorrhea (CIA).
MATERIALS AND METHODS
We retrospectively reviewed the data of breast patients treated between October 1999 and November 2007 without CIA. The Kaplan-Meier method was used for calculation of the survival rate. Log rank method and Cox regression analysis were used for univariate and multivariate prognostic analysis.
RESULTS
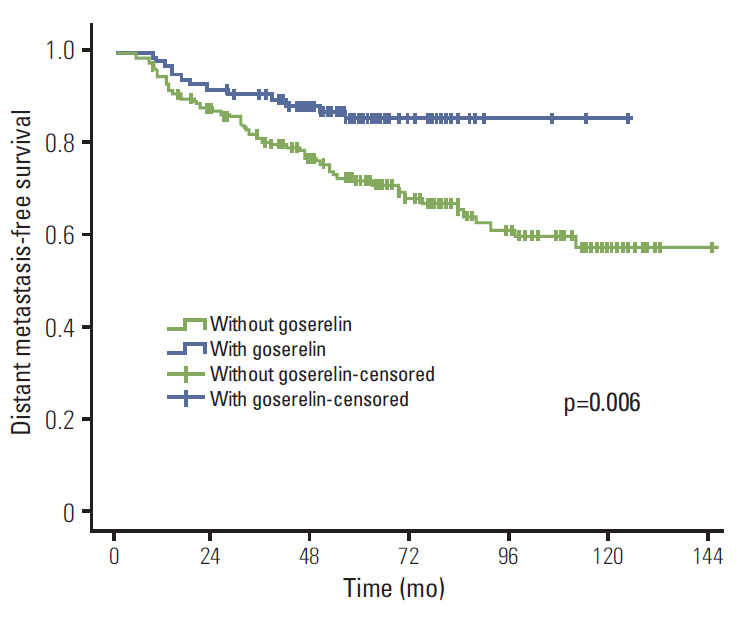
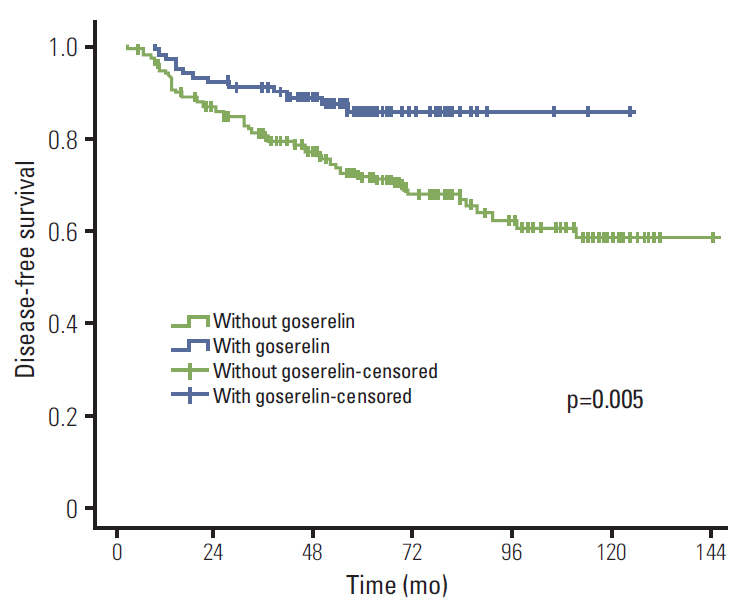
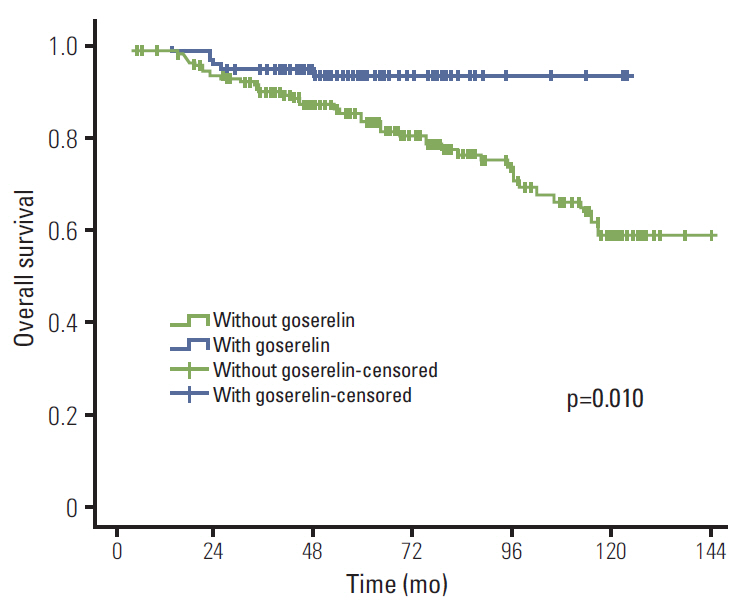
The median follow-up period was 61 months. Initially, 353 patients remained without CIA after chemotherapy and 98 among those who received goserelin and tamoxifen (TAM). In univariate analysis, goserelin improved locoregional recurrence-free survival (LRFS) (98.9% vs. 94.1%, p=0.041), distant metastasis-free survival (DMFS) (85.4% vs. 71.9%, p=0.006), disease-free survival (DFS) (85.4% vs. 71.6%, p=0.005), and overall survival (OS) (93.5% vs. 83.5%, p=0.010). In multivariate analysis, goserelin treatment was an independent factor influencing DMFS (hazard ratio [HR], 1.603; 95% confidence interval [CI], 1.228 to 2.092; p=0.001), DFS (HR, 1.606; 95% CI, 1.231 to 2.096; p=0.001), and OS (HR, 3.311; 95% CI, 1.416 to 7.742; p=0.006). In addition, treatment with goserelin resulted in significantly improved LRFS (p=0.039), DMFS (p=0.043), DFS (p=0.036), and OS (p=0.010) in patients aged < 40 years. In patients aged > or = 40 years, goserelin only improved DMFS (p=0.028) and DFS (p=0.027).
CONCLUSION
Ovarian ablation with goserelin plus TAM resulted in significantly improved therapeutic efficacy in premenopausal patients with stage II/III hormone receptor-positive breast cancer without CIA.
MeSH Terms
Figure
Reference
-
References
1. Li J, Zhang BN, Fan JH, Pang Y, Zhang P, Wang SL, et al. A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer. 2011; 11:364.
Article2. Han JG, Jiang YD, Zhang CH, Pang D, Zhang M, Wang YB, et al. Clinicopathologic characteristics and prognosis of young patients with breast cancer. Breast. 2011; 20:370–2.
Article3. Peng R, Wang S, Shi Y, Liu D, Teng X, Qin T, et al. Patients 35 years old or younger with operable breast cancer are more at risk for relapse and survival: a retrospective matched case-control study. Breast. 2011; 20:568–73.
Article4. Tan SH, Wolff AC. Luteinizing hormone-releasing hormone agonists in premenopausal hormone receptor-positive breast cancer. Clin Breast Cancer. 2007; 7:455–64.
Article5. Clarke MJ. Ovarian ablation in breast cancer, 1896 to 1998: milestones along hierarchy of evidence from case report to Cochrane review. BMJ. 1998; 317:1246–8.6. LHRH-agonists in Early Breast Cancer Overview Group, Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, et al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007; 369:1711–23.7. Wu S, Li Q, Zhu Y, Sun J, Li F, Lin H, et al. Role of goserelin in combination with endocrine therapy for the treatment of advanced breast cancer in premenopausal women positive for hormone receptor: a retrospective matched case-control study. Cancer Biother Radiopharm. 2013; 28:697–702.
Article8. Davidson NE, O'Neill AM, Vukov AM, Osborne CK, Martino S, White DR, et al. Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101(E5188). J Clin Oncol. 2005; 23:5973–82.9. Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009; 360:679–91.
Article10. Adjuvant Breast Cancer Trials Collaborative Group. Ovarian ablation or suppression in premenopausal early breast cancer: results from the international adjuvant breast cancer ovarian ablation or suppression randomized trial. J Natl Cancer Inst. 2007; 99:516–25.11. Aebi S, Davidson T, Gruber G, Cardoso F; ESMO Guidelines Working Group. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011; 22 Suppl 6:vi12–24.
Article12. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.13. Kaufmann M, Graf E, Jonat W, Eiermann W, Vescia S, Geberth M, et al. A randomised trial of goserelin versus control after adjuvant, risk-adapted chemotherapy in premenopausal patients with primary breast cancer - GABG-IV B-93. Eur J Cancer. 2007; 43:2351–8.
Article14. Sverrisdottir A, Johansson H, Johansson U, Bergh J, Rotstein S, Rutqvist L, et al. Interaction between goserelin and tamoxifen in a prospective randomised clinical trial of adjuvant endocrine therapy in premenopausal breast cancer. Breast Cancer Res Treat. 2011; 128:755–63.
Article15. Cheng TF, Wang JD, Uen WC. Cost-utility analysis of adjuvant goserelin (Zoladex) and adjuvant chemotherapy in premenopausal women with breast cancer. BMC Cancer. 2012; 12:33.
Article16. Karlsson P, Sun Z, Braun D, Price KN, Castiglione-Gertsch M, Rabaglio M, et al. Long-term results of International Breast Cancer Study Group Trial VIII: adjuvant chemotherapy plus goserelin compared with either therapy alone for premenopausal patients with node-negative breast cancer. Ann Oncol. 2011; 22:2216–26.
Article17. Baum M, Hackshaw A, Houghton J, Rutqvist , Fornander T, Nordenskjold B, et al. Adjuvant goserelin in pre-menopausal patients with early breast cancer: results from the ZIPP study. Eur J Cancer. 2006; 42:895–904.
Article18. Pagani O, O'Neill A, Castiglione M, Gelber RD, Goldhirsch A, Rudenstam CM, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer. 1998; 34:632–40.
Article19. Goldhirsch A, Gelber RD, Yothers G, Gray RJ, Green S, Bryant J, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001; 30:44–51.
Article20. Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006; 24:5769–79.
Article21. Arriagada R, Le MG, Spielmann M, Mauriac L, Bonneterre J, Namer M, et al. Randomized trial of adjuvant ovarian suppression in 926 premenopausal patients with early breast cancer treated with adjuvant chemotherapy. Ann Oncol. 2005; 16:389–96.
Article22. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, Breast Cancer. Version 1, 2011 [Internet]. Fort Washington: National Comprehensive Cancer Network;[cited 2010 Nov 8]. Available from: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.23. Regan MM, Pagani O, Walley B, Torrisi R, Perez EA, Francis P, et al. Premenopausal endocrine-responsive early breast cancer: who receives chemotherapy? Ann Oncol. 2008; 19:1231–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Ovarian Function Suppression in Premenopausal Women with Early Breast Cancer
- Efficacy of Combined Aromatase Inhibitor and Luteinizing Hormone-Releasing Hormone Agonist in Premenopausal Metastatic Breast Cancer
- The Examination of Ovarian Reserve in Premenopausal Patients with Hormone Receptor Positive Breast Cancer
- The Incidence of Chemotherapy-induced Amenorrhea and Recovery in Young (<45-year-old) Breast Cancer Patients
- Durable Response of Androgen Receptor-Positive Male Breast Cancer to Goserelin