J Breast Cancer.
2019 Mar;22(1):141-148. 10.4048/jbc.2019.22.e2.
Durable Response of Androgen Receptor-Positive Male Breast Cancer to Goserelin
- Affiliations
-
- 1Department of Clinical Oncology, Kasr Alainy School of Medicine, Cairo University, Cairo, Egypt. Loay.kassem@cairocure.com
- 2Department of Clinical Oncology, Cairo Oncology Center, Cairo, Egypt.
- 3Department of Pathology, Kasr Alainy School of Medicine, Cairo University, Cairo, Egypt.
- KMID: 2441859
- DOI: http://doi.org/10.4048/jbc.2019.22.e2
Abstract
- The luteinizing hormone-releasing hormone/androgen receptor (LHRH/AR) pathway is a promising treatment target in a subgroup of female patients with triple-negative breast cancer (TNBC). However, very little is known about the efficacy of this strategy in male patients with TNBC. In this report, we describe a male patient with AR-positive TNBC who was successfully treated using an LHRH agonist after pretreatment with several lines of chemotherapy and achieved a durable response. We also review the existing evidence supporting LHRH- and AR-targeted therapy for this rare disease.
MeSH Terms
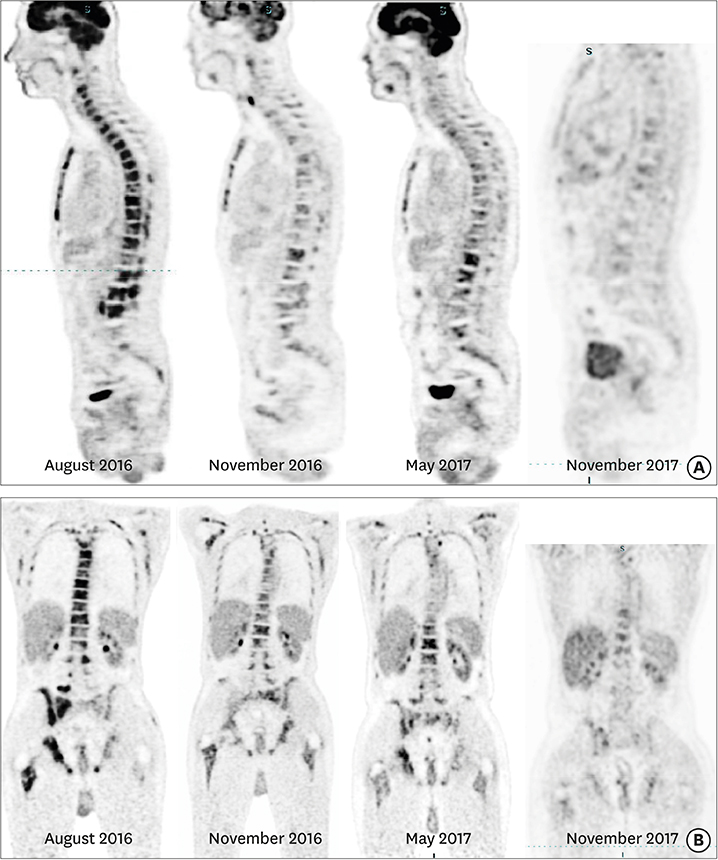
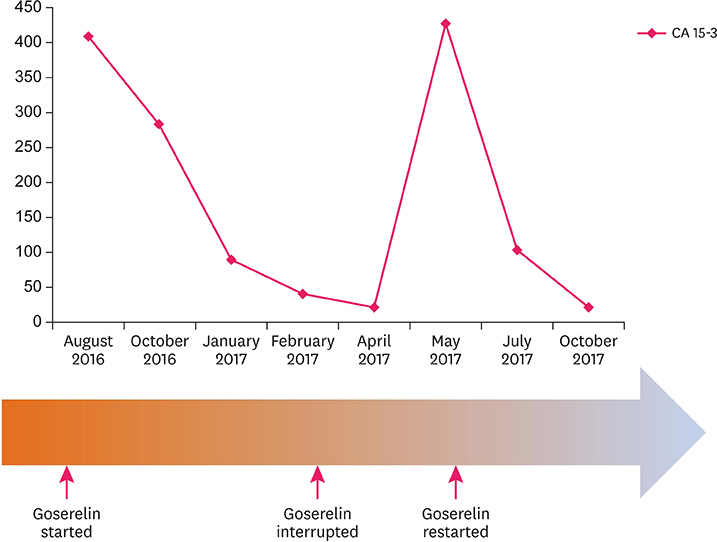
Figure
Reference
-
1. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011; 121:2750–2767.
Article2. Barton VN, D'Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015; 14:769–778.
Article3. Cardoso F, Bartlett JM, Slaets L, van Deurzen CH, van Leeuwen-Stok E, Porter P, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018; 29:405–417.
Article4. Di Lauro L, Barba M, Pizzuti L, Vici P, Sergi D, Di Benedetto A, et al. Androgen receptor and antiandrogen therapy in male breast cancer. Cancer Lett. 2015; 368:20–25.
Article5. Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013; 19:5505–5512.
Article6. Arce-Salinas C, Riesco-Martinez MC, Hanna W, Bedard P, Warner E. Complete response of metastatic androgen receptor-positive breast cancer to bicalutamide: case report and review of the literature. J Clin Oncol. 2016; 34:e21–e24.
Article7. Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O'Shaughnessy J, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018; 36:884–890.
Article8. Parker JS, Peterson AC, Tudor IC, Hoffman J, Uppal H.. A novel biomarker to predict sensitivity to enzalutamide (ENZA) in TNBC. J Clin Oncol. 2015; 33:1083.
Article9. Labrie F, Dupont A, Bélanger A, Lacourcière Y, Béland L, Cusan L, et al. Complete response to combination therapy with an LHRH agonist and flutamide in metastatic male breast cancer: a case report. Clin Invest Med. 1990; 13:275–278.10. Di Lauro L, Vici P, Barba M, Pizzuti L, Sergi D, Rinaldi M, et al. Antiandrogen therapy in metastatic male breast cancer: results from an updated analysis in an expanded case series. Breast Cancer Res Treat. 2014; 148:73–80.
Article11. Seitz S, Buchholz S, Schally AV, Weber F, Klinkhammer-Schalke M, Inwald EC, et al. Triple negative breast cancers express receptors for LHRH and are potential therapeutic targets for cytotoxic LHRH-analogs, AEZS 108 and AEZS 125. BMC Cancer. 2014; 14:847.
Article12. Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015; 372:923–932.
Article13. Kim HJ, Yoon TI, Chae HD, Kim JE, Chae EY, Yu JH, et al. Concurrent gonadotropin-releasing hormone agonist administration with chemotherapy improves neoadjuvant chemotherapy responses in young premenopausal breast cancer patients. J Breast Cancer. 2015; 18:365–370.
Article14. Lambertini M, Ceppi M, Poggio F, Peccatori FA, Azim HA Jr, Ugolini D, et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol. 2015; 26:2408–2419.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intermittent Androgen Deprivation with Goserelin and Flutamide for Prostate Cancer: a Pilot Study
- Clinical Significance of DAX-1 Expression using Immunihistochemical Staining in Breast Cancer
- Efficacy of Combined Aromatase Inhibitor and Luteinizing Hormone-Releasing Hormone Agonist in Premenopausal Metastatic Breast Cancer
- Androgen Receptor as a Predictive Marker for Pathologic Complete Response in Hormone Receptor–Positive and HER-2–Negative Breast Cancer with Neoadjuvant Chemotherapy
- Immunohistochemical Assay of Androgen Receptor in Prostate Cancer