Chonnam Med J.
2017 May;53(2):161-167. 10.4068/cmj.2017.53.2.161.
MicroRNA-29 Family Suppresses the Invasion of HT1080 Human Fibrosarcoma Cells by Regulating Matrix Metalloproteinase 2 Expression
- Affiliations
-
- 1Department of Microbiology and Immunology, Chonnam National University Medical School, Gwangju, Korea. bashin@jnu.ac.kr
- 2Department of Biomedical Sciences, Center for Creative Biomedical Scientists, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2379290
- DOI: http://doi.org/10.4068/cmj.2017.53.2.161
Abstract
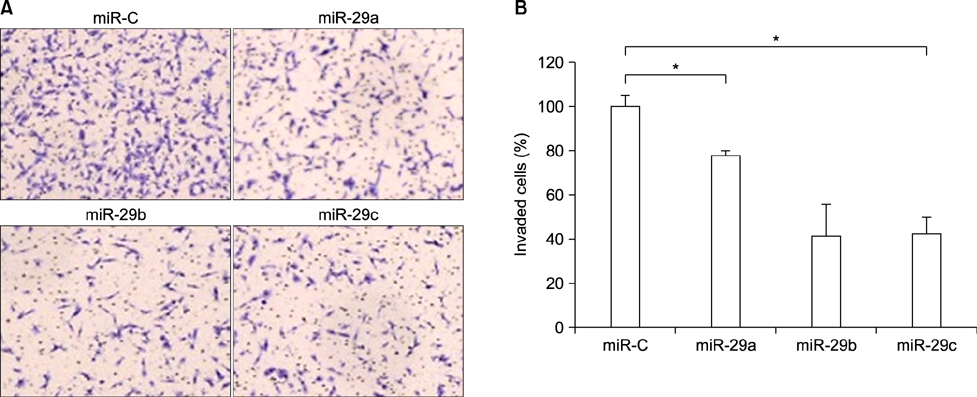
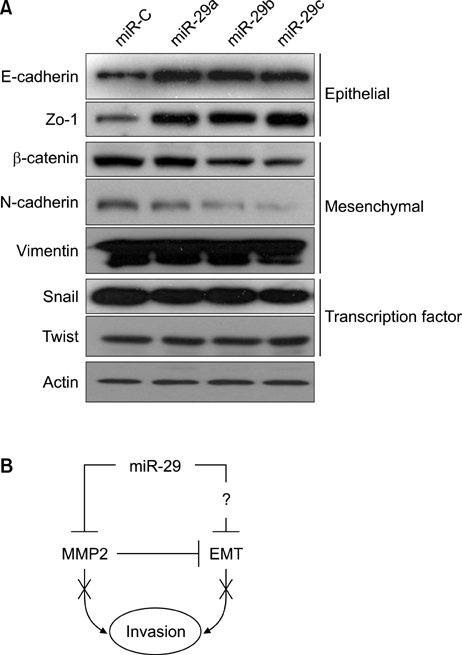
- Matrix metalloproteinase 2 (MMP2) is a potent protumorigenic, proangiogenic, and prometastatic enzyme that is overexpressed in metastatic cancer. Although there have been various studies on the MMP2 gene, further studies of regulatory factors are required to achieve inhibition of MMP2 enzyme activities. MicroRNAs (miRNAs) play key roles in tumor metastasis. However, the specific functions of miRNAs in metastasis are unclear. In this study, we assessed the function of the microRNA-29 family (miR-29s) in HT1080 human fibrosarcoma cells and examined the regulatory mechanisms of these miRNAs on MMP2 activation. Using miRanda, TargetScan, and PicTar databases, miR-29s were identified as candidate miRNAs targeting MMP2. Gain-of-function studies showed that overexpression of miR-29s could inhibit the invasion of HT1080 cells, suggesting their tumor-suppressive roles in HT1080 cells. In addition, dual luciferase reporter assays indicated that miR-29s could inhibit the expression of the luciferase gene containing the 3'-untranslated region of MMP2 mRNA. Ectopic expression of miR-29s down-regulated the expression of MMP2. Moreover, ectopic expression of miR-29s reduced MMP2 enzyme activity. These results suggested that miR-29s could decrease the invasiveness of HT1080 cells by modulating MMP2 signaling. Taken together, our results demonstrated that miR-29s may serve as therapeutic targets to control tumor metastasis.
MeSH Terms
Figure
Reference
-
1. Baranwal S, Alahari SK. miRNA control of tumor cell invasion and metastasis. Int J Cancer. 2010; 126:1283–1290.
Article2. Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008; 29:290–308.
Article3. Kerkelä E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003; 12:109–125.
Article4. Amălinei C, Căruntu ID, Bălan RA. Biology of metalloproteinases. Rom J Morphol Embryol. 2007; 48:323–334.5. Strongin AY, Marmer BL, Grant GA, Goldberg GI. Plasma membrane-dependent activation of the 72-kDa type IV collagenase is prevented by complex formation with TIMP-2. J Biol Chem. 1993; 268:14033–14039.
Article6. Roeb E, Matern S. Matrix metalloproteinases and colorectal cancer. Med Klin (Munich). 2003; 98:763–770.7. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003; 92:827–839.8. Li L, Li H. Role of microRNA-mediated MMP regulation in the treatment and diagnosis of malignant tumors. Cancer Biol Ther. 2013; 14:796–805.
Article9. Nassar FJ, Nasr R, Talhouk R. MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol Ther. 2017; 172:34–49.
Article10. Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007; 8:93–103.
Article11. Garzon R, Heaphy CE, Havelange V, Fabbri M, Volinia S, Tsao T, et al. MicroRNA 29b functions in acute myeloid leukemia. Blood. 2009; 114:5331–5341.
Article12. Chou J, Lin JH, Brenot A, Kim JW, Provot S, Werb Z. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat Cell Biol. 2013; 15:201–213.
Article13. Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007; 104:15805–15810.
Article14. Fang JH, Zhou HC, Zeng C, Yang J, Liu Y, Huang X, et al. MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology. 2011; 54:1729–1740.
Article15. Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009; 16:23–29.
Article16. Varshney J, Subramanian S. MicroRNAs as potential target in human bone and soft tissue sarcoma therapeutics. Front Mol Biosci. 2015; 2:31.
Article17. Liu P, Wilson MJ. miR-520c and miR-373 upregulate MMP9 expression by targeting mTOR and SIRT1, and activate the Ras/Raf/MEK/Erk signaling pathway and NF-ĸB factor in human fibrosarcoma cells. J Cell Physiol. 2012; 227:867–876.
Article18. Weng C, Dong H, Chen G, Zhai Y, Bai R, Hu H, et al. miR-409-3p inhibits HT1080 cell proliferation, vascularization and metastasis by targeting angiogenin. Cancer Lett. 2012; 323:171–179.
Article19. Hurst DR, Edmonds MD, Welch DR. Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res. 2009; 69:7495–7498.
Article20. Li L, Xiao B, Tong H, Xie F, Zhang Z, Xiao GG. Regulation of breast cancer tumorigenesis and metastasis by miRNAs. Expert Rev Proteomics. 2012; 9:615–625.
Article21. Ru P, Steele R, Newhall P, Phillips NJ, Toth K, Ray RB. miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol Cancer Ther. 2012; 11:1166–1173.
Article22. Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002; 295:2387–2392.
Article23. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000; 18:1135–1149.
Article24. Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009; 28:15–33.
Article25. Doerner AM, Zuraw BL. TGF-beta1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1beta but not abrogated by corticosteroids. Respir Res. 2009; 10:100.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Membrane-type Matrix Metalloproteinase-1 Induced Invasive and Angiogenic Activities in Chick Chorioallantoic Membrane (CAM) Model
- Antisense Deoxyoligonucleotides Inhibit Activities of Matrix Metalloproteinase-2 in Human Fibrosarcoma HT1080 Cells
- Acer mono Extract Inhibits Invasive Activities and G1/S Transition of HT1080 Fibrosarcoma Cells
- Novel Suppressive Effects of Ketotifen on Migration and Invasion of MDA-MB-231 and HT-1080 Cancer Cells
- The Effect of the Genistein on the Proliferation of HT1080 and Expression of Membrane Type 1-Matrix Metalloproteinase (MT1-MMP) mRNA