Allergy Asthma Immunol Res.
2017 Jul;9(4):347-359. 10.4168/aair.2017.9.4.347.
Usefulness of In Vivo and In Vitro Diagnostic Tests in the Diagnosis of Hypersensitivity Reactions to Quinolones and in the Evaluation of Cross-Reactivity: A Comprehensive Study Including the Latest Quinolone Gemifloxacin
- Affiliations
-
- 1Division of Immunology and Allergic Diseases, Department of Internal Medicine, Istanbul University, Istanbul Faculty of Medicine, Istanbul, Turkey. ertansemra@yahoo.com
- 2Department of Immunology, Institute of Experimental Medicine (DETAE), Istanbul University, Istanbul, Turkey.
- KMID: 2378198
- DOI: http://doi.org/10.4168/aair.2017.9.4.347
Abstract
- PURPOSE
Reports evaluating diagnosis and cross reactivity of quinolone hypersensitivity have revealed contradictory results. Furthermore, there are no reports investigating the cross-reactivity between gemifloxacin (GFX) and the others. We aimed to detect the usefulness of diagnostic tests of hypersensitivity reactions to quinolones and to evaluate the cross reactivity between different quinolones including the latest quinolone GFX.
METHODS
We studied 54 patients (mean age 42.31±10.39 years; 47 female) with 57 hypersensitivity reactions due to different quinolones and 10 nonatopic quinolone tolerable control subjects. A detailed clinical history, skin test (ST), and single-blind placebo-controlled drug provocation test (SBPCDPT), as well as basophil activation test (BAT) and lymphocyte transformation test (LTT) were performed with the culprit and alternative quinolones including ciprofloxacin (CFX), moxifloxacin (MFX), levofloxacin (LFX), ofloxacin (OFX), and GFX.
RESULTS
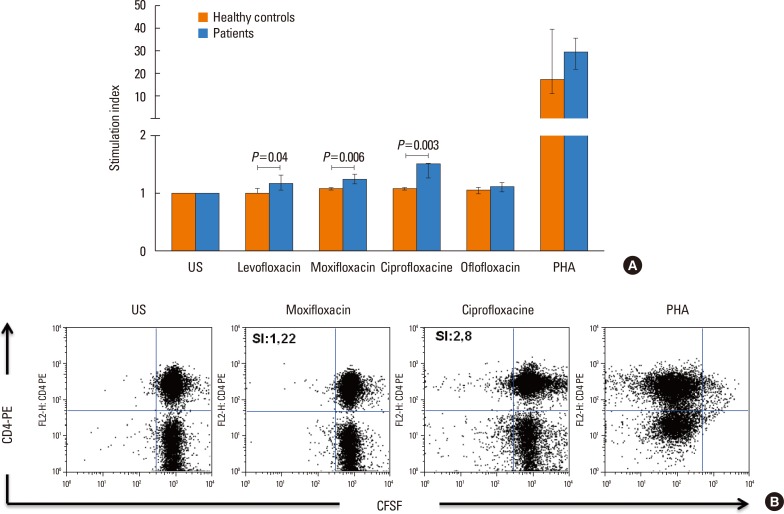
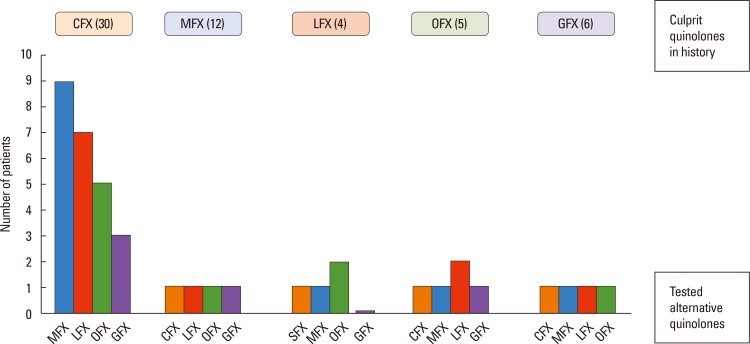
The majority (75.9%) of the patients reported immediate type reactions to various quinolones. The most common culprit drug was CFX (52.6%) and the most common reaction type was urticaria (26.3%). A quarter of the patients (24.1%) reacted to SBPCDPTs, although their STs were negative; while false ST positivity was 3.5% and ST/SBPCDPTs concordance was only 1.8%. Both BAT and LTT were not found useful in quinolone hypersensitivity. Cross-reactivity was primarily observed between LFX and OFX (50.0%), whereas it was the least between MFX and the others, and in GFX hypersensitive patients the degree of cross-reactivity to the other quinolones was 16.7%.
CONCLUSIONS
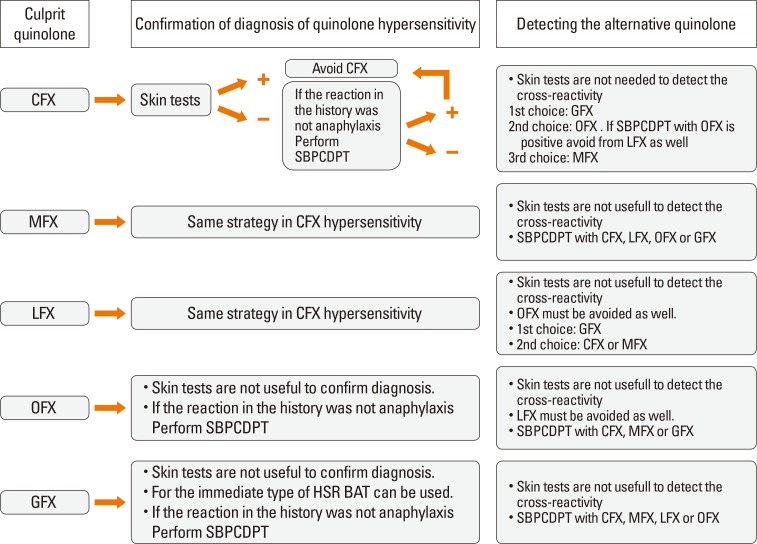
These results suggest that STs, BAT, and LTT are not supportive in the diagnosis of a hypersensitivity reaction to quinolone as well as in the prediction of cross-reactivity. Drug provocation tests (DPTs) are necessary to identify both culprit and alternative quinolones.
MeSH Terms
Figure
Reference
-
1. Van Bambeke F, Michot JM, Van Eldere J, Tulkens PM. Quinolones in 2005: an update. Clin Microbiol Infect. 2005; 11:256–280. PMID: 15760423.
Article2. Ball P. Quinolone generations: natural history or natural selection? J Antimicrob Chemother. 2000; 46:17–24. PMID: 10997595.
Article3. Bertino J Jr, Fish D. The safety profile of the fluoroquinolones. Clin Ther. 2000; 22:798–817. PMID: 10945507.
Article4. Doña I, Blanca-López N, Torres MJ, García-Campos J, García-Núñez I, Gómez F, et al. Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012; 22:363–371.5. Sachs B, Riegel S, Seebeck J, Beier R, Schichler D, Barger A, et al. Fluoroquinolone-associated anaphylaxis in spontaneous adverse drug reaction reports in Germany: differences in reporting rates between individual fluoroquinolones and occurrence after first-ever use. Drug Saf. 2006; 29:1087–1100.6. Venturini Díaz M, Lobera Labairu T, del Pozo Gil MD, Blasco Sarramián A, González Mahave I. In vivo diagnostic tests in adverse reactions to quinolones. J Investig Allergol Clin Immunol. 2007; 17:393–398.7. Blanca-López N, Andreu I, Torres Jaén MJ. Hypersensitivity reactions to quinolones. Curr Opin Allergy Clin Immunol. 2011; 11:285–291. PMID: 21659860.
Article8. Genlicik A, Özşeker ZF, Çolakoğlu B, Dal M, Büyüköztürk S. Hypersensitivity reactions due to antibiotics: the significance of provocation tests in determining alternative antibiotics. Asthma Allergy Immunol. 2013; 11:23–31.9. González I, Lobera T, Blasco A, del Pozo MD. Immediate hypersensitivity to quinolones: moxifloxacin cross-reactivity. J Investig Allergol Clin Immunol. 2005; 15:146–149.10. González-Mancebo E, Fernández-Rivas M. Immediate hypersensitivity to levofloxacin diagnosed through skin prick test. Ann Pharmacother. 2004; 38:354. PMID: 14742782.
Article11. Sánchez-Morillas L, Rojas Pérez-Ezquerra P, Reaño-Martos M, Laguna-Martínez JJ, Gómez-Tembleque P. Systemic anaphylaxis caused by moxifloxacin. Allergol Immunopathol (Madr). 2010; 38:226–227. PMID: 20089346.
Article12. Lobera T, Audícana MT, Alarcón E, Longo N, Navarro B, Muñoz D. Allergy to quinolones: low cross-reactivity to levofloxacin. J Investig Allergol Clin Immunol. 2010; 20:607–611.13. Aranda A, Mayorga C, Ariza A, Doña I, Rosado A, Blanca-Lopez N, et al. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy. 2011; 66:247–254. PMID: 20722637.
Article14. Seitz CS, Bröcker EB, Trautmann A. Diagnostic testing in suspected fluoroquinolone hypersensitivity. Clin Exp Allergy. 2009; 39:1738–1745. PMID: 19735271.
Article15. Scherer K, Bircher AJ. Hypersensitivity reactions to fluoroquinolones. Curr Allergy Asthma Rep. 2005; 5:15–21. PMID: 15659258.
Article16. Manfredi M, Severino M, Testi S, Macchia D, Ermini G, Pichler WJ, et al. Detection of specific IgE to quinolones. J Allergy Clin Immunol. 2004; 113:155–160. PMID: 14713922.
Article17. Empedrad R, Darter AL, Earl HS, Gruchalla RS. Nonirritating intradermal skin test concentrations for commonly prescribed antibiotics. J Allergy Clin Immunol. 2003; 112:629–630. PMID: 13679828.
Article18. Perez E, Callero A, Martinez-Tadeo JA, Hernandez G, Rodríguez-Plata E, Almeida Z, et al. Are skin tests useful in fluoroquinolone hypersensitivity diagnosis? Ann Allergy Asthma Immunol. 2013; 111:423–425.
Article19. Ben Said B, Berard F, Bienvenu J, Nicolas JF, Rozieres A. Usefulness of basophil activation tests for the diagnosis of IgE-mediated allergy to quinolones. Allergy. 2010; 65:535–536. PMID: 19845576.
Article20. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004; 59:809–820. PMID: 15230812.
Article21. Dávila I, Diez ML, Quirce S, Fraj J, De La Hoz B, Lazaro M. Cross-reactivity between quinolones. Report of three cases. Allergy. 1993; 48:388–390. PMID: 8368469.22. Schmid DA, Depta JP, Pichler WJ. T cell-mediated hypersensitivity to quinolones: mechanisms and cross-reactivity. Clin Exp Allergy. 2006; 36:59–69. PMID: 16393267.
Article23. Fukushima K, Nakatsubo M, Noda M, Uenami T, Hayama Y, Tsuruta N, et al. Anaphylaxis due to intravenous levofloxacin with tolerance to garenoxacin. Intern Med. 2012; 51:1769–1772. PMID: 22790143.
Article24. Chang B, Knowles SR, Weber E. Immediate hypersensitivity to moxifloxacin with tolerance to ciprofloxacin: report of three cases and review of the literature. Ann Pharmacother. 2010; 44:740–745. PMID: 20233910.
Article25. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International Consensus on drug allergy. Allergy. 2014; 69:420–437. PMID: 24697291.
Article26. Simons FE, Ardusso LR, BilòM B, El-Gamal YM, Ledford DK, Ring J, et al. World Allergy Organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011; 4:13–37. PMID: 23268454.
Article27. Rouzaire P, Nosbaum A, Denis L, Bienvenu F, Bérard F, Cozon G, et al. Negativity of the basophil activation test in quinolone hypersensitivity: a breakthrough for provocation test decision-making. Int Arch Allergy Immunol. 2012; 157:299–302. PMID: 22041525.
Article28. Mailing HJ. 2. Methods of skin testing. Allergy. 1993; 48(Suppl 14):55–56.
Article29. Barbaud A, Gonçalo M, Bruynzeel D, Bircher A. European Society of Contact Dermatitis. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001; 45:321–328. PMID: 11846746.30. Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003; 58:854–863. PMID: 12911412.
Article31. Kulthanan K, Chularojanamontri L, Manapajon A, Dhana N, Jongjarearnprasert K. Cutaneous adverse reactions to fluoroquinolones. Dermatitis. 2011; 22:155–160. PMID: 21569745.
Article32. Blanca-López N, Ariza A, Doña I, Mayorga C, Montañez MI, Garcia-Campos J, et al. Hypersensitivity reactions to fluoroquinolones: analysis of the factors involved. Clin Exp Allergy. 2013; 43:560–567. PMID: 23600547.
Article33. Campi P, Pichler WJ. Quinolone hypersensitivity. Curr Opin Allergy Clin Immunol. 2003; 3:275–281. PMID: 12865771.
Article34. Choi IS, Han ER, Lim SW, Lim SR, Kim JN, Park SY, et al. Beta-lactam antibiotic sensitization and its relationship to allergic diseases in tertiary hospital nurses. Allergy Asthma Immunol Res. 2010; 2:114–122. PMID: 20358025.
Article35. Sánchez-Borges M, Capriles-Hulett A. Atopy is a risk factor for nonsteroidal anti-inflammatory drug sensitivity. Ann Allergy Asthma Immunol. 2000; 84:101–106. PMID: 10674573.36. Kim SY, Kim JH, Jang YS, Choi JH, Park S, Hwang YI, et al. The basophil activation test is safe and useful for confirming drug-induced anaphylaxis. Allergy Asthma Immunol Res. 2016; 8:541–544. PMID: 27582406.
Article37. Gómez E, Torres MJ, Mayorga C, Blanca M. Immunologic evaluation of drug allergy. Allergy Asthma Immunol Res. 2012; 4:251–263. PMID: 22950030.
Article38. Fernández TD, Ariza A, Palomares F, Montañez MI, Salas M, Martín-Serrano A, et al. Hypersensitivity to fluoroquinolones: the expression of basophil activation markers depends on the clinical entity and the culprit fluoroquinolone. Medicine (Baltimore). 2016; 95:e3679. PMID: 27281069.39. Mangodt EA, Van Gasse AL, Decuyper I, Uyttebroek A, Faber MA, Sabato V, et al. In vitro diagnosis of immediate drug hypersensitivity: should we go with the flow. Int Arch Allergy Immunol. 2015; 168:3–12. PMID: 26524156.40. Dewachter P, Mouton-Faivre C. Anaphylaxis to levofloxacin. Allergol Immunopathol (Madr). 2013; 41:418–419. PMID: 23265267.
Article41. Saravolatz LD, Leggett J. Gatifloxacin, gemifloxacin, and moxifloxacin: the role of 3 newer fluoroquinolones. Clin Infect Dis. 2003; 37:1210–1215. PMID: 14557966.
Article42. Anti-Infective Drugs Advisors Committee (US). Factive (Gemifloxacin): U.S. Food and Drug Administration briefing document. Silver Spring (MD): U.S. Food and Drug Administration;2003. 3. 04.43. Ball P, Mandell L, Patou G, Dankner W, Tillotson G. A new respiratory fluoroquinolone, oral gemifloxacin: a safety profile in context. Int J Antimicrob Agents. 2004; 23:421–429. PMID: 15120718.
Article44. Yilmaz I, Doğan S, Tutar N, Kanbay A, Büyükoğlan H, Demir R. Biphasic anaphylaxis to gemifloxacin. Asia Pac Allergy. 2012; 2:280–282. PMID: 23130335.
Article45. Shear N. Gemifloxacin: cutaneous manifestations (FDA Advisory Committee Meeting March 4, 2003) [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;2003. 2003 March 4. Available from: www.fda.gov/ohrms/dockets/ac/03/slides/3931S1_04_LFLife%20Sciences-Factive.pdf.