Cancer Res Treat.
2017 Apr;49(2):399-407. 10.4143/crt.2016.215.
Predictive Value of Tertiary Lymphoid Structures Assessed by High Endothelial Venule Counts in the Neoadjuvant Setting of Triple-Negative Breast Cancer
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. backlila@gmail.com
- 2Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2378111
- DOI: http://doi.org/10.4143/crt.2016.215
Abstract
- PURPOSE
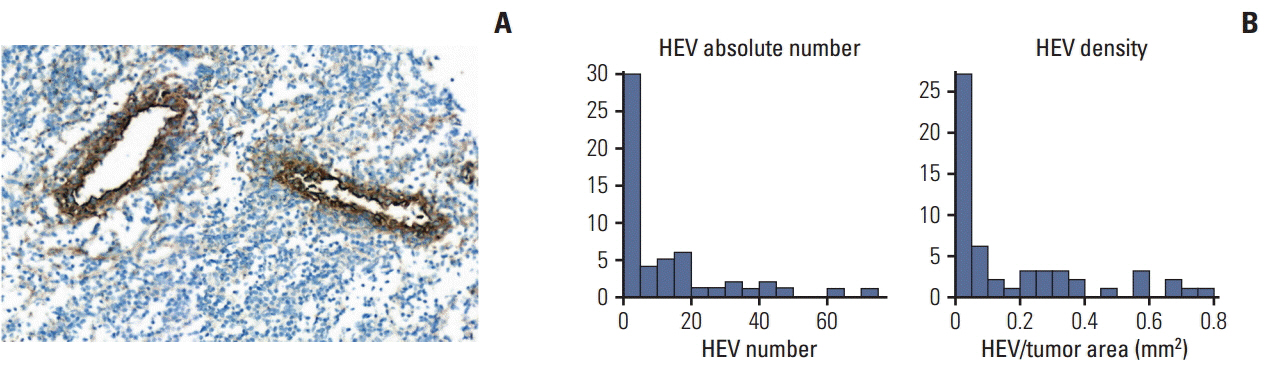
The tertiary lymphoid structure (TLS) is an important source of tumor-infiltrating lymphocytes (TILs), which have a strong prognostic and predictive value in triple-negative breast cancer (TNBC). A previous study reported that the levels of CXCL13 mRNA expression were associated with TLSs, but measuring the gene expression is challenging in routine practice. Therefore, this study evaluated the MECA79-positive high endothelial venule (HEV) densities and their association with the histopathologically assessed TLSs in biopsy samples. In addition, the relationship of TLSs with the CXCL13 transcript levels and clinical outcomes were examined.
MATERIALS AND METHODS
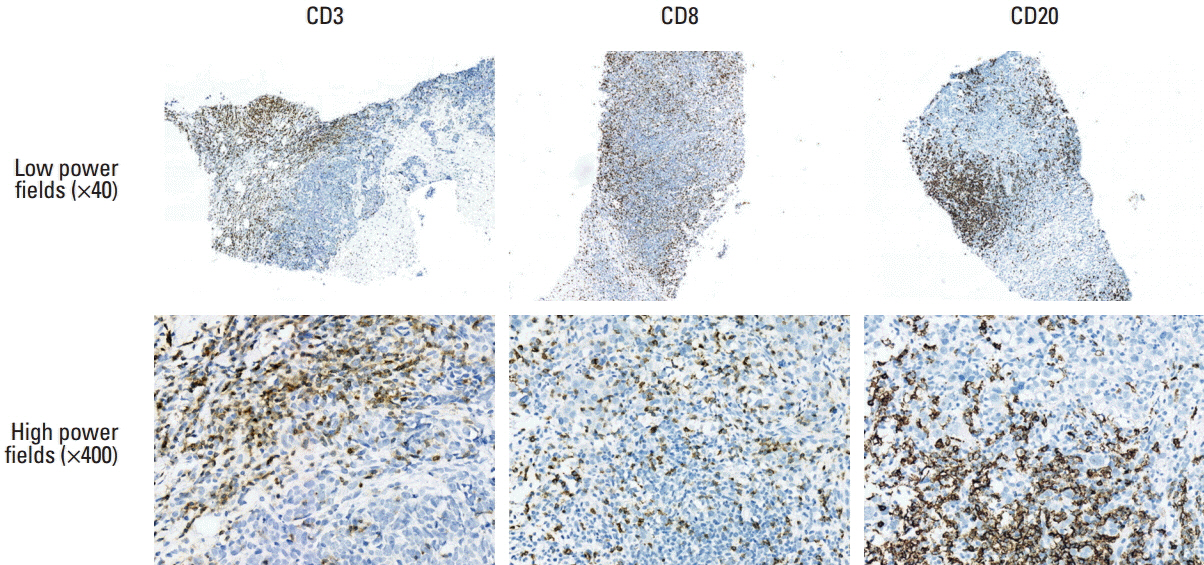
A total of 108 TNBC patients treated with neoadjuvant chemotherapy (NAC) were studied. The amounts of TILs and TLSs were measured histopathologically using hematoxylin and eosin-stained slides. The HEV densities and TIL subpopulations were measured by immunohistochemistry for MECA79, CD3, CD8, and CD20. CXCL13mRNA expression levels using a NanoString assay (NanoString Technologies).
RESULTS
The mean number of HEVs in pre-NAC biopsies was 12 (range, 0 to 72). The amounts of TILs and TLSs, HEV density, and CXCL13 expression showed robust correlations with each other. A lower pre-NAC clinical T stage, higher TIL and TLS levels, a higher HEV density, CD20-positive cell density, and CXCL13 expression were significant predictors of a pathologic complete response (pCR). Higher CD8-positive cell density and levels of CXCL13 expression were significantly associated with a better disease-free survival rate.
CONCLUSION
MECA79-positive HEV density in pre-NAC biopsies is an objective and quantitative surrogate marker of TLS and might be a valuable tool for predicting pCR of TNBC in routine pathology practice.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Lymphocyte-Activation Gene-3 Expression and Prognostic Value in Neoadjuvant-Treated Triple-Negative Breast Cancer
Yunxuan Wang, Tieying Dong, Qijia Xuan, Hong Zhao, Ling Qin, Qingyuan Zhang
J Breast Cancer. 2018;21(2):124-133. doi: 10.4048/jbc.2018.21.2.124.The Association of Estrogen Receptor Activity, Interferon Signaling, and MHC Class I Expression in Breast Cancer
In Hye Song, Young-Ae Kim, Sun-Hee Heo, Won Seon Bang, Hye Seon Park, Yeon Ho Choi, Heejae Lee, Jeong-Han Seo, Youngjin Cho, Sung Wook Jung, Hee Jeong Kim, Sei Hyun Ahn, Hee Jin Lee, Gyungyub Gong
Cancer Res Treat. 2022;54(4):1111-1120. doi: 10.4143/crt.2021.1017.
Reference
-
References
1. Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992; 28A:859–64.
Article2. Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996; 77:1303–10.
Article3. Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G. Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013; 16:32–9.
Article4. Ahn SG, Jeong J, Hong S, Jung WH. Current Issues and clinical evidence in tumor-infiltrating lymphocytes in breast cancer. J Pathol Transl Med. 2015; 49:355–63.
Article5. West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011; 13:R126.
Article6. Stagg J, Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol. 2013; 5:169–81.
Article7. Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011; 71:5678–87.
Article8. Lee HJ, Kim JY, Park IA, Song IH, Yu JH, Ahn JH, et al. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol. 2015; 144:278–88.
Article9. Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, et al. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology. 2012; 1:829–39.10. Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012; 12:762–73.
Article11. Ladanyi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, et al. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007; 56:1459–69.
Article12. Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008; 26:4410–7.
Article13. Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014; 20:2147–58.
Article14. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013; 123:2873–92.
Article15. Lee HJ, Lee JJ, Song IH, Park IA, Kang J, Yu JH, et al. Prognostic and predictive value of NanoString-based immune-related gene signatures in a neoadjuvant setting of triple-negative breast cancer: relationship to tumor-infiltrating lymphocytes. Breast Cancer Res Treat. 2015; 151:619–27.
Article16. Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012; 33:297–305.
Article17. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011; 29:1949–55.
Article18. Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012; 14:R48.
Article19. Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008; 68:5405–13.
Article20. Brown JR, Wimberly H, Lannin DR, Nixon C, Rimm DL, Bossuyt V. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 2014; 20:5995–6005.
Article21. Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007; 25:4414–22.
Article22. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015; 26:259–71.
Article23. Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008; 26:317–25.
Article24. Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol. 2013; 3:231.
Article25. Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat. 2012; 132:545–53.
Article26. Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013; 109:2705–13.
Article27. Kang H, Cheong H, Cho MS, Koo H, Han WS, Lee KE, et al. Significance of Foxp3 positive regulatory T cell and tumor infiltrating T lymphocyte in triple negative breast cancer. Korean J Pathol. 2011; 45:53–61.
Article28. Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010; 185:4977–82.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- Potential of Cells and Cytokines/Chemokines to Regulate Tertiary Lymphoid Structures in Human Diseases
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Recent Advances in the Neoadjuvant Treatment of Breast Cancer
- Fear of Cancer Recurrence and Unmet Needs in Triple Negative Breast Cancer Survivors