J Korean Med Sci.
2017 Jun;32(6):1042-1045. 10.3346/jkms.2017.32.6.1042.
DEND Syndrome with Heterozygous KCNJ11 Mutation Successfully Treated with Sulfonylurea
- Affiliations
-
- 1Department of Pediatrics, University of Ulsan College of Medicine, Asan Medical Center Children's Hospital, Seoul, Korea. hwyoo@amc.seoul.kr
- 2Medical Genetics Center, University of Ulsan College of Medicine, Asan Medical Center Children's Hospital, Seoul, Korea.
- KMID: 2377737
- DOI: http://doi.org/10.3346/jkms.2017.32.6.1042
Abstract
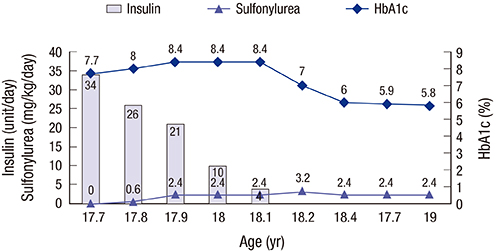
- Permanent neonatal diabetes mellitus (PNDM) is caused by mutations in the ATP-sensitive potassium channel (K(ATP) channel) subunits. Developmental delay, epilepsy, and neonatal diabetes (DEND) syndrome is the most severe form of PNDM and is characterized by various neurologic features. We report on a patient with DEND syndrome following initial misdiagnosis with type 1 DM, who was successfully switched from insulin to sulfonylurea therapy. A 50-day-old male presented with fever and seizure, complicated by persistent hyperglycemia. Insulin therapy was initiated. At 10 months of age, the patient was unable to hold his head up and make eye contact with others. At 17.9 years of age, direct sequencing of KCNJ11 identified a heterozygous mutation of c.602G>A (p.R201H). Since then, treatment with gliclazide was initiated and the insulin dose was gradually reduced. Following 3 months, insulin was discontinued with a gliclazide dose of 2.4 mg/kg. The patient continued to have excellent glycemic control with a glycated hemoglobin (HbA1c) level of 5.8% after 5 months. However, the patient's psychomotor retardation did not improve. This study reports the first case of DEND syndrome in Korea caused by a KCNJ11 mutation and emphasizes the necessity to screen mutations in KATP channel genes in patients with neonatal diabetes.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Successful switching from insulin to sulfonylurea in a 3-month-old infant with diabetes due to p.G53D mutation in
KCNJ11
Jong Seo Yoon, Kyu Jung Park, Young Bae Sohn, Hae Sang Lee, Jin Soon Hwang
Ann Pediatr Endocrinol Metab. 2018;23(3):154-157. doi: 10.6065/apem.2018.23.3.154.
Reference
-
1. Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006; 355:467–477.2. Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, Aguilar-Bryan L, Gagel RF, Bryan J. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995; 268:426–429.3. Nestorowicz A, Inagaki N, Gonoi T, Schoor KP, Wilson BA, Glaser B, Landau H, Stanley CA, Thornton PS, Seino S, et al. A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes. 1997; 46:1743–1748.4. Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004; 350:1838–1849.5. Shimomura K, Hörster F, de Wet H, Flanagan SE, Ellard S, Hattersley AT, Wolf NI, Ashcroft F, Ebinger F. A novel mutation causing DEND syndrome: a treatable channelopathy of pancreas and brain. Neurology. 2007; 69:1342–1349.6. Shimomura K. The K(ATP) channel and neonatal diabetes. Endocr J. 2009; 56:165–175.7. Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005; 54:2503–2513.8. Ahn SY, Kim GH, Yoo HW. Successful sulfonylurea treatment in a patient with permanent neonatal diabetes mellitus with a novel KCNJ11 mutation. Korean J Pediatr. 2015; 58:309–312.9. Kim MS, Kim SY, Kim GH, Yoo HW, Lee DW, Lee DY. Sulfonylurea therapy in two Korean patients with insulin-treated neonatal diabetes due to heterozygous mutations of the KCNJ11 gene encoding Kir6.2. J Korean Med Sci. 2007; 22:616–620.10. Sperling MA. ATP-sensitive potassium channels--neonatal diabetes mellitus and beyond. N Engl J Med. 2006; 355:507–510.11. Jones AG, Hattersley AT. Reevaluation of a case of type 1 diabetes mellitus diagnosed before 6 months of age. Nat Rev Endocrinol. 2010; 6:347–351.12. Temple IK, Gardner RJ, Mackay DJ, Barber JC, Robinson DO, Shield JP. Transient neonatal diabetes: widening the understanding of the etiopathogenesis of diabetes. Diabetes. 2000; 49:1359–1366.13. Vaxillaire M, Populaire C, Busiah K, Cavé H, Gloyn AL, Hattersley AT, Czernichow P, Froguel P, Polak M. Kir6.2 mutations are a common cause of permanent neonatal diabetes in a large cohort of French patients. Diabetes. 2004; 53:2719–2722.14. Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006; 49:1190–1197.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful sulfonylurea treatment in a patient with permanent neonatal diabetes mellitus with a novel KCNJ11 mutation
- Successful switching from insulin to sulfonylurea in a 3-month-old infant with diabetes due to p.G53D mutation in KCNJ11
- Neonatal Diabetes Mellitus Due to KCNJ11 (KIR6.2) Mutation Successfully Treated with Sulfonylurea
- Sulfonylurea Therapy in Two Korean Patients with Insulin-treated Neonatal Diabetes due to Heterozygous Mutations of the KCNJ11 Gene Encoding Kir6.2
- A Case of Transient Neonatal Diabetes Mellitus Attributable to a Nonspecific Mutation in the ABCC8 Gene