J Korean Med Sci.
2007 Aug;22(4):616-620. 10.3346/jkms.2007.22.4.616.
Sulfonylurea Therapy in Two Korean Patients with Insulin-treated Neonatal Diabetes due to Heterozygous Mutations of the KCNJ11 Gene Encoding Kir6.2
- Affiliations
-
- 1Department of Pediatrics, Chonbuk National University Medical School, Jeonju, Korea. leedy@chonbuk.ac.kr
- 2Research Institute of Clinical Medicine, Chonbuk National University Medical School, Jeonju, Korea.
- 3Medical Genetics Clinics and Labortory, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pediatrics, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Medicine, Soonchunhyang University, Seoul, Korea.
- KMID: 1127075
- DOI: http://doi.org/10.3346/jkms.2007.22.4.616
Abstract
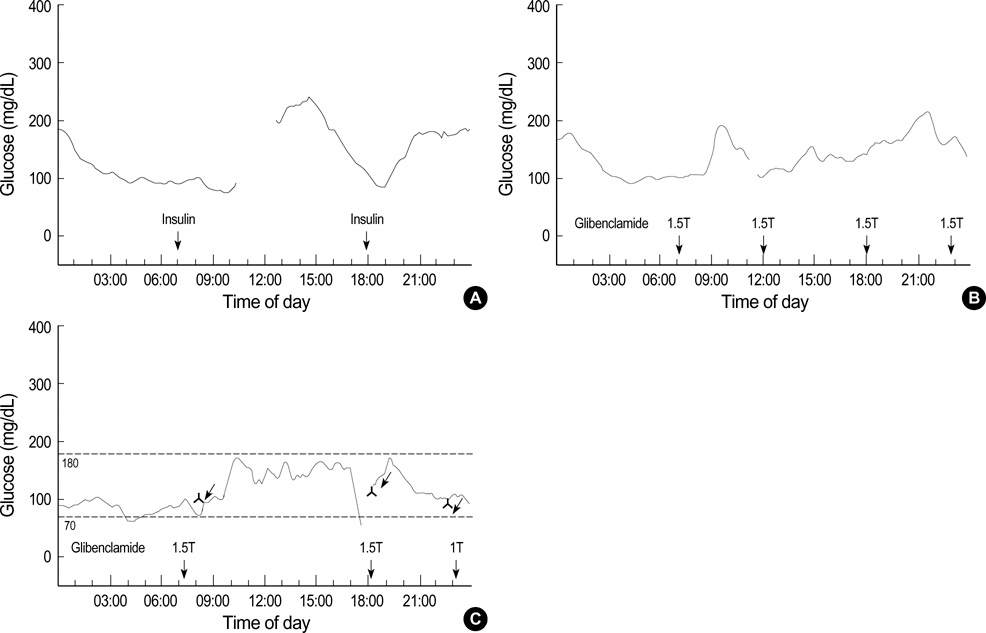
- Permanent neonatal diabetes (PND) is a rare form of diabetes characterized by insulin-requiring hyperglycemia diagnosed within the first three months of life. In most cases, the causes are not known. Recently, mutations in the KCNJ11 gene encoding the Kir6.2 subunit of the ATP-sensitive K(+) channel have been described in patients with PND. We report the first two Korean cases with PND due to a lysineto- arginine substitution at position 170 (K179R) and a valine-to-methionine substitution at position 59 (V59M) mutations of KCNJ11 encoding Kir6.2, respectively. After several years of insulin therapy, these patients were managed by oral glibenclamide therapy at a daily dose of 0.8-0.9 mg/kg. Their basal c-peptide levels increased after one week of glibenclamide therapy, and one month later, the insulin and c-peptide levels were in the normal ranges without any episodes of hyper- or hypoglycemia. These cases demonstrate that oral sulfonylurea may be the treatment of choice in PND patients with KCNJ11 mutations even at a young age.
Keyword
MeSH Terms
-
Base Sequence
C-Peptide/blood
DNA Mutational Analysis
Diabetes Mellitus/blood/*drug therapy/genetics
Female
Glyburide/*therapeutic use
Hemoglobin A, Glycosylated/metabolism
Heterozygote
Humans
Hypoglycemic Agents/therapeutic use
Infant
Infant, Newborn
Insulin/blood/*therapeutic use
Korea
*Mutation
Potassium Channels, Inwardly Rectifying/*genetics
Sulfonylurea Compounds/therapeutic use
Treatment Outcome
Figure
Cited by 1 articles
-
Successful switching from insulin to sulfonylurea in a 3-month-old infant with diabetes due to p.G53D mutation in
KCNJ11
Jong Seo Yoon, Kyu Jung Park, Young Bae Sohn, Hae Sang Lee, Jin Soon Hwang
Ann Pediatr Endocrinol Metab. 2018;23(3):154-157. doi: 10.6065/apem.2018.23.3.154.
Reference
-
1. Shield JP. Neonatal diabetes: new insights into aetiology and implications. Horm Res. 2000. 53:Suppl 1. 7–11.
Article2. Temple IK, Gardner RJ, Mackay DJ, Barber JC, Robinson DO, Shield JP. Transient neonatal diabetes:widening the understanding of the etiopathogenesis of diabetes. Diabets. 2000. 49:1359–1366.3. Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, Frayling TM, Temple IK, Mackay D, Shield JP, Sumnki Z, van Rhijn A, Wales JK, Clark P, Gorman S, Aisenberg J, Ellard S, Njolstad PR, Ashcroft FM, Hattersley AT. Activating mutations in the gene encoding the ATP-sensitive potassium channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004. 350:1838–1849.4. Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995. 270:1166–1170.
Article5. Aschcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984. 312:446–448.6. Gribble FM, Reimann F. Sulfonylurea action revisited: the post-cloning era. Diabetelogia. 2003. 46:875–891.7. Sagen JV, Raeder H, Eba H, Naim S, Kolbeinn G, Halvor B, Abuelo D, Phornphutkul C, Molnes J, Bell GI, Gloyn AL, Hattersley AT, Molven A, Sovik O, Njolstad PR. Permanent Neonatal Diabetes due to Mutations in KCNJ11 Encoding Kir6.2: Patient characteristics and initial response to sulfonylurea therapy. Diabetes. 2004. 53:2713–2718.8. Hattersley AT, Ashcroft FM. Activating Mutations in Kir6.2 and neonatal diabetes: New clinical syndromes, new scientific insights and new therapy. Diabetes. 2005. 54:2503–2513.
Article9. Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutation in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006. 49:1190–1197.10. Suzuki S, Mukai T, Matsuo K, Ueda O, Ito Y, Makita Y, Fujieda K. KCNJ11 gene activating mutations in Japanese patients with neonatal diabetes mellitus. Horm Research. 2005. 64:Suppl 1. 136.11. Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, Temple IK, Mackay DJ, Shield JP, Freedenberg D, Noyes K, Ellard S, Ashcroft FM, Gribble FM, Hattersley AT. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005. 14:925–934.12. Yorifuji T, Nagashima K, Kurokawa K, Kawai M, Oishi M, Akazawa Y, Hosokawa M, Yamada Y, Inagaki N, Nakahata T. The C42R mutation in the Kir6.2 (KCNJ11) gene as a cause of transient neonatal diabetes, childhood diabetes, or later-onset, apparently type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005. 90:3174–3178.13. Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, Ashcroft FM. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci USA. 2004. 101:17539–17544.
Article14. Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001. 27:18–20.
Article15. Zung A, Glaser B, Nimri R, Zadik Z. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J Clin Endocrinol Metab. 2004. 89:5504–5507.
Article16. Codner E, Flanagan S, Ellard S, Garcia H, Hattersley A. High-dose glibenclamide can replace insulin therapy despite transitory diarrhea in early-onset diabetes caused by a novel R201L Kir6.2 mutation. Diabetes Care. 2005. 28:758–759.
Article17. Klupa T, Edghill EL, Nazim J, Sieradzki J, Ellard S, Hattersley AT, Malecki MT. The identification of a R201H mutation in KCNJ11, which encodes Kir6.2, and successful transfer to sustained-release sulphonylurea therapy in a subject with neonatal diabetes: evidence for heterogeneity of beta cell function among carriers of the R201H mutation. Diabetologia. 2005. 48:1029–1031.18. Trapp S, Proks P, Tucker SJ, Ashcroft FM. Molecular analysis of ATP-sensitive K channel gating and implications for channel inhibition by ATP. J Gen Physiol. 1998. 112:333–349.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful sulfonylurea treatment in a patient with permanent neonatal diabetes mellitus with a novel KCNJ11 mutation
- Neonatal Diabetes Mellitus Due to KCNJ11 (KIR6.2) Mutation Successfully Treated with Sulfonylurea
- Successful switching from insulin to sulfonylurea in a 3-month-old infant with diabetes due to p.G53D mutation in KCNJ11
- DEND Syndrome with Heterozygous KCNJ11 Mutation Successfully Treated with Sulfonylurea
- Neonatal Diabetes Caused by Activating Mutations in the Sulphonylurea Receptor