Lab Anim Res.
2017 Mar;33(1):40-47. 10.5625/lar.2017.33.1.40.
HemoHIM, a herbal preparation, alleviates airway inflammation caused by cigarette smoke and lipopolysaccharide
- Affiliations
-
- 1College of Veterinary Medicine (BK21 Plus Project Team), Chonnam National University, Gwangju, Korea. dvmmk79@gmail.com
- 2Natural Product Research Center, Korea Research Institute of Bioscience and Biotechnology, Jeongeup, Korea.
- KMID: 2375271
- DOI: http://doi.org/10.5625/lar.2017.33.1.40
Abstract
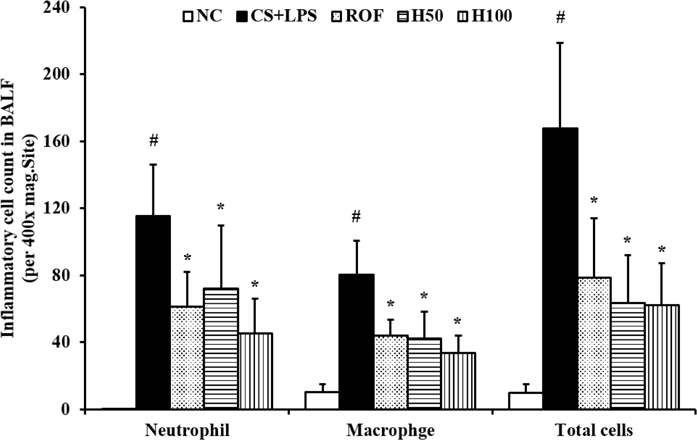
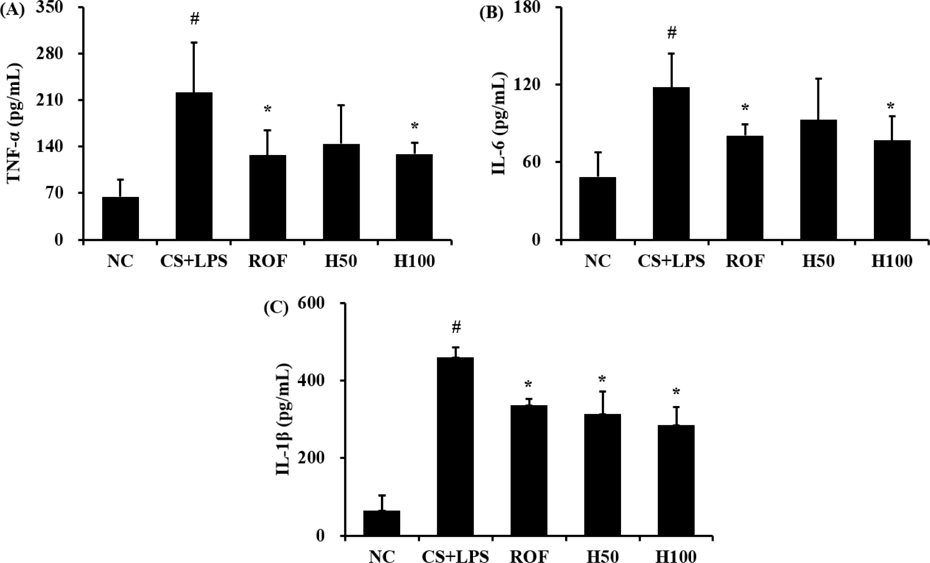
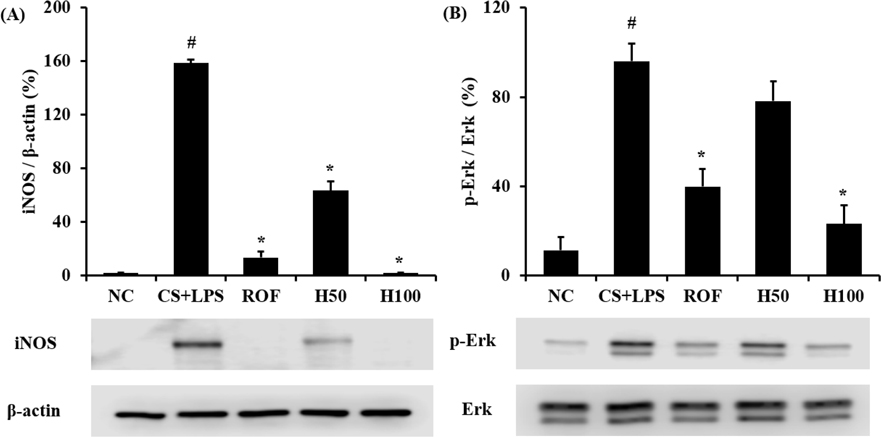
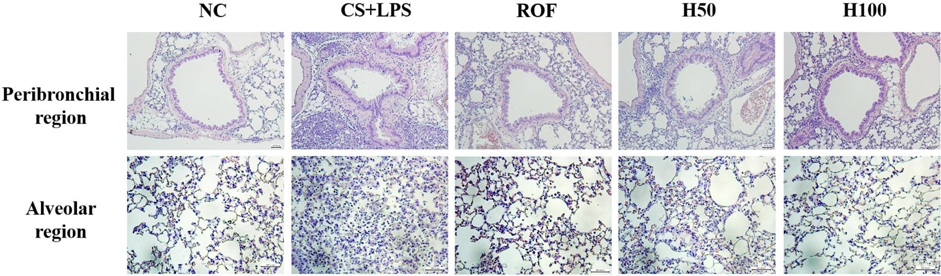
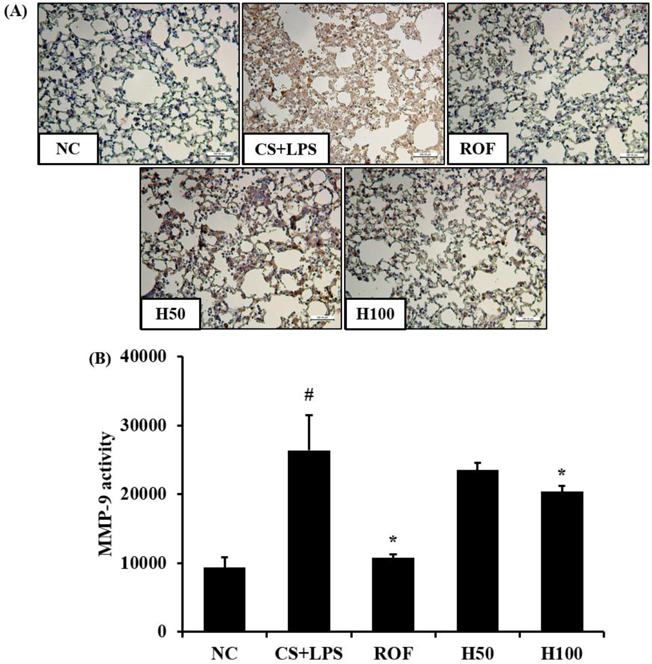
- HemoHIM, herbal preparation has designed for immune system recovery. We investigated the anti-inflammatory effect of HemoHIM on cigarette smoke (CS) and lipopolysaccharide (LPS) induced chronic obstructive pulmonary disease (COPD) mouse model. To induce COPD, C57BL/6 mice were exposed to CS for 1 h per day (eight cigarettes per day) for 4 weeks and intranasally received LPS on day 26. HemoHIM was administrated to mice at a dose of 50 or 100 mg/kg 1h before CS exposure. HemoHIM reduced the inflammatory cell count and levels of tumor necrosis factor receptor (TNF)-α, interleukin (IL)-6 and IL-1β in the broncho-alveolar lavage fluid (BALF) induced by CS+LPS exposure. HemoHIM decreased the inflammatory cell infiltration in the airway and inhibited the expression of iNOS and MMP-9 and phosphorylation of Erk in lung tissue exposed to CS+LPS. In summary, our results indicate that HemoHIM inhibited a reduction in the lung inflammatory response on CS and LPS induced lung inflammation via the Erk pathway. Therefore, we suggest that HemoHIM has the potential to treat pulmonary inflammatory disease such as COPD.
MeSH Terms
-
Animals
Cell Count
Immune System
Inflammation*
Interleukins
Lung
MAP Kinase Signaling System
Matrix Metalloproteinase 9
Mice
Phosphorylation
Plant Preparations*
Pneumonia
Pulmonary Disease, Chronic Obstructive
Receptors, Tumor Necrosis Factor
Smoke*
Therapeutic Irrigation
Tobacco Products*
Interleukins
Matrix Metalloproteinase 9
Plant Preparations
Receptors, Tumor Necrosis Factor
Smoke
Figure
Reference
-
1. Tang W, Shen Z, Guo J, Sun S. Screening of long non-coding RNA and TUG1 inhibits proliferation with TGF-induction in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016; 11:2951–2964.2. Korpershoek Y, Vervoort S, Nijssen L, Trappenburg J, Schuurmans MJ. Factors influencing exacerbation-related self-management in patients with COPD: a qualitative study. Int J Chron Obstruct Pulmon Dis. 2016; 11:2977–2990.3. Boehme SA, Franz-Bacon K, Ludka J, DiTirro DN, Ly TW, Bacon KB. MAP3K19 Is Overexpressed in COPD and Is a Central Mediator of Cigarette Smoke-Induced Pulmonary Inflammation and Lower Airway Destruction. PLoS One. 2016; 11(12):e0167169.4. Yang J, Yu HM, Zhou XD, Huang HP, Han Zh, Kolosov VP, Perelman JM. Cigarette smoke induces mucin hypersecretion and inflammatory response through the p66shc adaptor protein-mediated mechanism in human bronchial epithelial cells. Mol Immunol. 2016; 69:86–98.5. Lin K, Liu S, Shen Y, Li Q. Berberine attenuates cigarette smoke-induced acute lung inflammation. Inflammation. 2013; 36(5):1079–1086.6. Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. 2012; 91(2):142–149.7. Xue WH, Shi XQ, Liang SH, Zhou L, Liu KF, Zhao J. Emodin Attenuates Cigarette Smoke Induced Lung Injury in a Mouse Model via Suppression of Reactive Oxygen Species Production. J Biochem Mol Toxicol. 2015; 29(11):526–532.8. Ge LT, Liu YN, Lin XX, Shen HJ, Jia YL, Dong XW, Sun Y, Xie QM. Inhalation of ambroxol inhibits cigarette smoke-induced acute lung injury in a mouse model by inhibiting the Erk pathway. Int Immunopharmacol. 2016; 33:90–98.9. Schmitt KR, Diestel A, Lehnardt S, Schwartlander R, Lange PE, Berger F, Ullrich O, Abdul-Khaliq H. Hypothermia suppresses inflammation via ERK signaling pathway in stimulated microglial cells. J Neuroimmunol. 2007; 189(1-2):7–16.10. Shin IS, Ahn KS, Shin NR, Jeon CM, Kwon OK, Chin YW, Lee K, Oh SR. Homoegonol attenuates the asthmatic responses induced by ovalbumin challenge. Arch Pharm Res. 2014; 37(9):1201–1210.11. Cho A, Graves J, Reidy MA. Mitogen-activated protein kinases mediate matrix metalloproteinase-9 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000; 20(12):2527–2532.12. Lee IT, Yang CM. Inflammatory signalings involved in airway and pulmonary diseases. Mediators Inflamm. 2013; 2013:791231.13. Doz E, Noulin N, Boichot E, Guénon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, Couillin I. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008; 180(2):1169–1178.14. Park HR, Jo SK, Jung U, Yee ST. Restoration of the immune functions in aged mice by supplementation with a new herbal composition, HemoHIM. Phytother Res. 2008; 22(1):36–42.15. Jo SK, Lee HJ, Kim SR, Kim JC, Bae CS, Jung U, Park HR, Jang JS, Kim SH. Antiinflammatory activity of an herbal preparation (HemoHIM) in rats. Phytother Res. 2007; 21(7):625–628.16. Park HR, Jo SK, Choi NH, Jung U. HemoHIM ameliorates the persistent down-regulation of Th1-like immune responses in fractionated γ-irradiated mice by modulating the IL-12p70-STAT4 signaling pathway. Radiat Res. 2012; 177(5):676–684.17. Kim JJ, Cho HW, Park HR, Jung U, Jo SK, Yee ST. Preventative effect of an herbal preparation (HemoHIM) on development of airway inflammation in mice via modulation of Th1/2 cells differentiation. PLoS One. 2013; 8(7):e68552.18. Kim JJ, Choi J, Lee MK, Kang KY, Paik MJ, Jo SK, Jung U, Park HR, Yee ST. Immunomodulatory and Antidiabetic Effects of a New Herbal Preparation (HemoHIM) on Streptozotocin-Induced Diabetic Mice. Evid Based Complement Alternat Med. 2014; 2014:461685.19. Shin IS, Shin NR, Park JW, Jeon CM, Hong JM, Kwon OK, Kim JS, Lee IC, Kim JC, Oh SR, Ahn KS. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J Pineal Res. 2015; 58(1):50–60.20. Lee H, Park JR, Kim EJ, Kim WJ, Hong SH, Park SM, Yang SR. Cigarette smoke-mediated oxidative stress induces apoptosis via the MAPKs/STAT1 pathway in mouse lung fibroblasts. Toxicol Lett. 2016; 240(1):140–148.21. Lee KH, Lee CH, Jeong J, Jang AH, Yoo CG. Neutrophil Elastase Differentially Regulates Interleukin 8 (IL-8) and Vascular Endothelial Growth Factor (VEGF) Production by Cigarette Smoke Extract. J Biol Chem. 2015; 290(47):28438–28445.22. Gernez Y, Tirouvanziam R, Chanez P. Neutrophils in chronic inflammatory airway diseases: can we target them and how? Eur Respir J. 2010; 35(3):467–469.23. Jeong SH, Park JH, Kim JN, Park YH, Shin SY, Lee YH, Kye YC, Son SW. Up-regulation of TNF-alpha secretion by cigarette smoke is mediated by Egr-1 in HaCaT human keratinocytes. Exp Dermatol. 2010; 19(8):e206–e212.24. Wang H, Yang T, Shen Y, Wan C, Li X, Li D, Liu Y, Wang T, Xu D, Wen F, Ying B. Ghrelin Inhibits Interleukin-6 Production Induced by Cigarette Smoke Extract in the Bronchial Epithelial Cell Via NF-κB Pathway. Inflammation. 2016; 39(1):190–198.25. Markovics JA, Araya J, Cambier S, Somanath S, Gline S, Jablons D, Hill A, Wolters PJ, Nishimura SL. Interleukin-1beta induces increased transcriptional activation of the transforming growth factor-beta-activating integrin subunit beta8 through altering chromatin architecture. J Biol Chem. 2011; 286(42):36864–36874.26. Cha SM, Cha JD, Jang EJ, Kim GU, Lee KY. Sophoraflavanone G prevents Streptococcus mutans surface antigen I/II-induced production of NO and PGE2 by inhibiting MAPK-mediated pathways in RAW 264.7 macrophages. Arch Oral Biol. 2016; 68:97–104.27. Grzela K, Litwiniuk M, Zagorska W, Grzela T. Airway Remodeling in Chronic Obstructive Pulmonary Disease and Asthma: the Role of Matrix Metalloproteinase-9. Arch Immunol Ther Exp (Warsz). 2016; 64(1):47–55.28. Jiang WT, Liu XS, Xu YJ, Ni W, Chen SX. Expression of Nitric Oxide Synthase Isoenzyme in Lung Tissue of Smokers with and without Chronic Obstructive Pulmonary Disease. Chin Med J (Engl). 2015; 128(12):1584–1589.29. Mercer PF, Shute JK, Bhowmik A, Donaldson GC, Wedzicha JA, Warner JA. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005; 6:151.30. Roh GS, Yi CO, Cho YJ, Jeon BT, Nizamudtinova IT, Kim HJ, Kim JH, Oh YM, Huh JW, Lee JH, Hwang YS, Lee SD, Lee JD. Anti-inflammatory effects of celecoxib in rat lungs with smoke-induced emphysema. Am J Physiol Lung Cell Mol Physiol. 2010; 299(2):L184–L191.31. Hesslinger C, Strub A, Boer R, Ulrich WR, Lehner MD, Braun C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans. 2009; 37(Pt 4):886–891.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective effect of water extract of guibi-tang against pulmonary inflammation induced by cigarette smoke and lipopolysaccharide
- Pathogenesis and pathophysiology of COPD
- Safety of a cigarette-type aid to stop smoking
- Acute eosinophilic pneumonia caused by passive smoking
- Coexisting Upper Airway Inflammation in Chronic Obstructive Pulmonary Disease: A Review of the Literature