Korean J Phys Anthropol.
2017 Mar;30(1):29-38. 10.11637/kjpa.2017.30.1.29.
CD44 Expression in Microglia of the Retina and Cerebellum of Developing and Adult Chicken
- Affiliations
-
- 1Department of Anatomy, College of Medicine, Chungbuk National University, Korea. eylee@chungbuk.ac.kr seojh@chungbuk.ac.kr
- KMID: 2374774
- DOI: http://doi.org/10.11637/kjpa.2017.30.1.29
Abstract
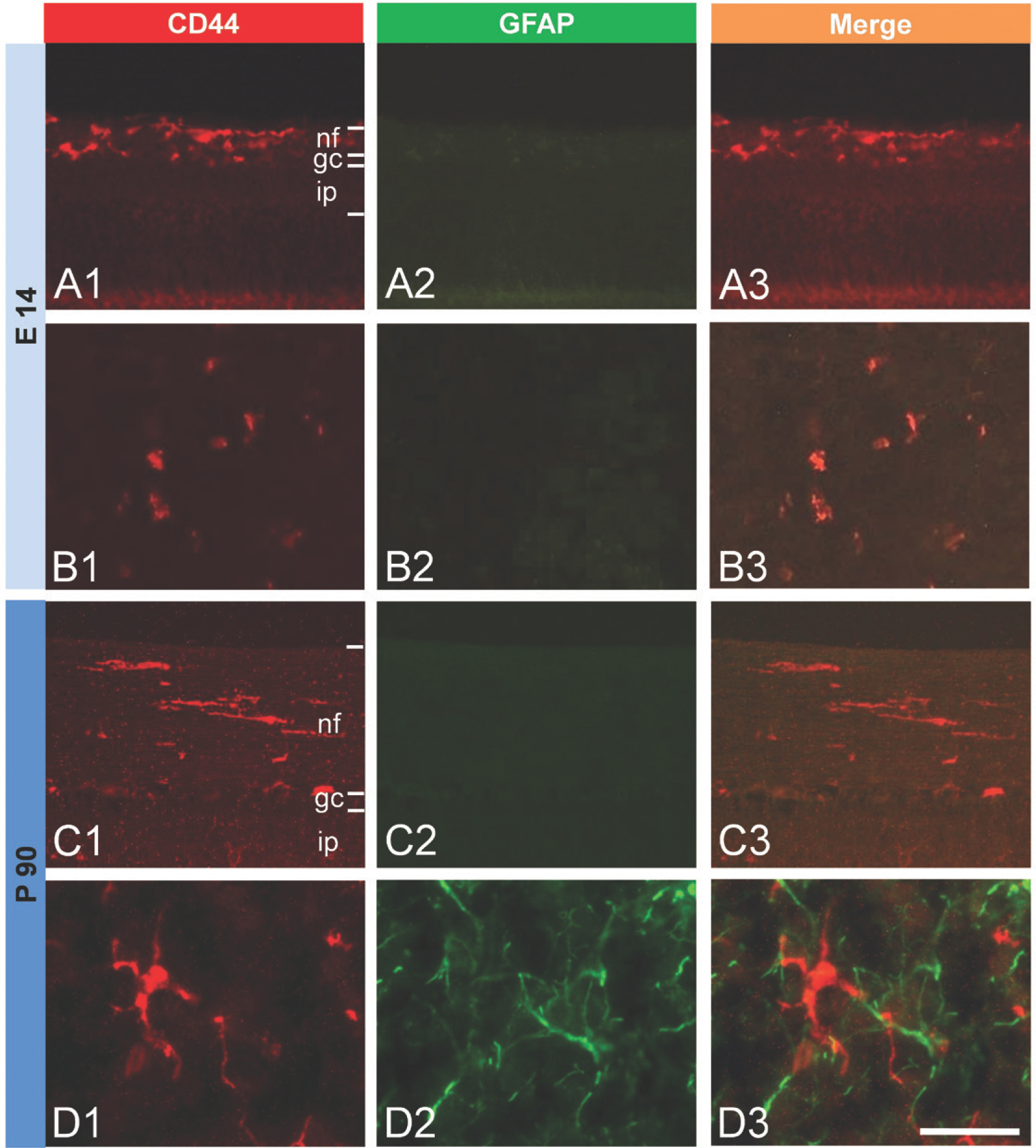
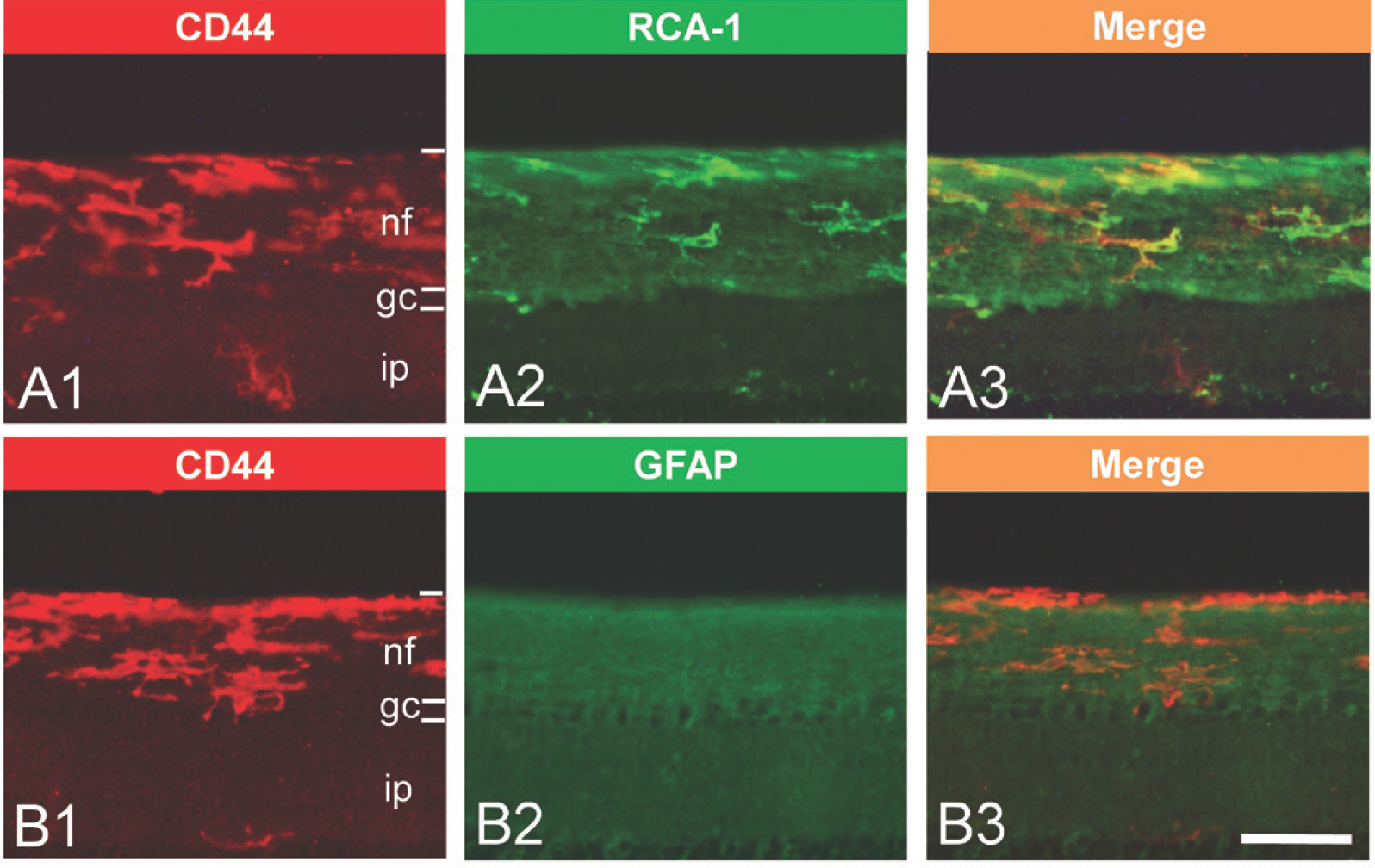
- CD44 is a transmembrane protein that acts as a receptor for an adhesion molecule, hyaluronic acid. The type of cells expressing CD44 and roles of CD44 are still controversial and need to be elucidated. The aim of the present study was to examine the type of cells expressing CD44 and the changes in their distribution in the retina and the cerebellum of the developing and adult chicken. Embryonic day 14 (E14) and post-hatch day 90 (P90) chickens were used in this study. CD44-immunoreactive (ir) cells were observed both in the retina and the cerebellum of the two developmental stages examined. In the retina of E14, CD44-ir cells were mainly located in the nerve fiber layer. In adults, most of the CD44-ir cells were in the nerve fiber layer and some were dispersed in other layers of the retina. In the cerebellum of E14, CD44-ir cells were distributed throughout the cerebellar cortex, including the external and internal granular layers. CD44-ir cells were more frequently found in the cerebellum of P90 adult chickens than in that of E14 embryos. At higher magnification, CD44-ir cells showed ramified cytoplasmic processes irradiating from their cell bodies. In the retina and in the cerebellum of all ages examined, double staining showed that most of the CD44-ir cells also expressed RCA-1, a marker of microglia. In contrast to that, at the same locations, GFAP and CD44 were not co-expressed in cells. When the adult retina was stimulated by LPS, CD44 immunoreactivity increased, and CD44-ir cells were also RCA-1-positive. The present results indicated that CD44 was expressed in microglia of the retina and the cerebellum of the developing and adult chicken even in normal conditions, and microglial CD44 expression was increased upon LPS stimulation.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990; 61:1303–13.
Article2. Ponta H, Sherman I, Herrlich PA. CD44: from adhesion molecules to signaling regulators. Nat Rev Mol Cell Biol. 2003; 4:33–45.3. Sherman LS, Back SA. A ‘GAG'reflex prevents repair of the damaged CNS. Trends Neurosci. 2008; 31:44–52.4. Corbel C, Lehmann A, Davison F. Expression CD44 during early development of the chick embryo. Mech Dev. 2000; 96:111–4.5. Alfei L, Aita M, Caronti B, Vita RD, Margotta V, Albani LM, et al. Hyaluronate receptor CD44 is expressed by astrocytes in the adult chicken and in astrocyte cell precursors in early development of the chick spinal cord. Eur J Histochem. 1999; 43:29–38.6. Liu Y, Han SSW, Wu Y, Tuohy TMF, Xue H, Cai J, et al. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2001; 276:31–46.
Article7. Cai N, Kurachi M, Shibasaki K, Okano-Uchida T, Ishizaki Y. CD44-positive cells are candidates for astrocytes precursor cells in developing mouse cerebellum. Cerebellum. 2011; 11:181–93.8. Baier C, Baader SL, Jankowski J, Gieselmann V, Schilling K, Rauch U, et al. Hyaluronan is organized into fiber-like structures along migratory pathways in the developing mouse cerebellum. Matrix Biol. 2007; 26:348–58.
Article9. Shinoe T, Kuribayashi H, Saya H, Seiki M, Aburatani H, Watanabe S. Identification of CD44 as a cell surface marker for Müller glia precursor cells. J Neurochem. 2010; 115:1633–42.
Article10. Wang H, Zhan Y, Xu L, Feuerstein GZ, Wang X. Use of suppression subtractive hybridization for differential gene expression in stroke: Discovery of CD44 gene expression and localization in permanent focal stroke in rats. Stroke. 2001; 32:1020–27.11. Wang X, Xu L, Wang H, Zhan Y, Puré E, Feuerstein GZ. CD44 deficiency in mice protects brain from cerebral ischemia injury. J Neurochem. 2001; 83:1172–79.
Article12. Jones LL, Liu Z, Shen J, Werner A, Kreutzberg GW, Raiv-ich G. Regulation of the cell adhesion molecule CD44 after nerve transaction and direct trauma to the mouse brain. J Comp Neurol. 2000; 23:468–92.13. Matsumoto T, Imagama S, Hirano K, Ohgomori T, Natori T, Kobayashi K, et al. CD44 expression in astrocytes and microglia is associated with ALS progression in a mouse model. Neurosci Lett. 2012; 520:115–20.
Article14. Kim S, Cho SH, Kim KY, Shin KY, Kim HS, Park CH, et al. Α-synuclein induces migration of BV-2 microglial cells by upregulation of CD44 and MT1-MMP. J Neurochem. 2009; 109:1483–96.
Article15. Gorlewicz A, Wlodarczyk J, Wilczek E, Gawlak M, Cabaj A, Majczynski H, et al. CD44 is expressed in non-myelin-ating Schwann cells of the adult rat, and may play a role in neurodegeneration-induced glial plasticity at the neuromuscular junction. Neurobiol Dis. 2009; 34:245–58.
Article16. Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engrafment. Nat Med. 2001; 7:1356–61.17. Vallieres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003; 23:5197–207.18. Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1992; 195:231–72.
Article19. Mannoji H, Yeger H, Becker LE. A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol. 1986; 71:341–3.20. Kim JY, Song SH, Kim HN, Kim DW, Sohn HJ, Lee EY, et al. αB-crystallin is expressed in myelinating oligodendro-cytes of the developing and adult avian retina. Neurochem Res. 2012; 37:2135–42.
Article21. Genini S, Beltran WA, Stein VM, Aguirre GD. Isolation and ex vivo characterizarion of the immunophenotype and function of microglia/macrophage populations in normal dog retina. Adv Exp Med Biol. 2014; 801:339–45.22. Alfei L, Aita M, Caronti B, Vita RD, Margotta V, Albani LM, et al. Hyaluronate receptor CD44 is expressed by astrocytes in the adult chicken and in astrocyte cell precursors in early development of the chick spinal cord. Eur J Histochem. 1999; 43:29–38.23. Naruse M, Shibasaki K, Yokoyama S, Kurachi M, Ishizaki Y. Dynamic changes of CD44 expression from progenitors to subpopulations of astrocytes and neurons in developing cerebellum. PLoS One. 2013; 8:1–12.
Article24. Buiani M, Postma N, Polder E, Dieleman N, Scheffer PG, Sim FJ, et al. Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. Brain. 2013; 136:209–22.25. Sherman LS, Struve JN, Rangwala R, Wallingford NM, Tuohy TMF, Iv CK. Hyaluronate-based extracellular matrix: keeping glia in their place. Glia. 2002; 38:93–102.
Article26. Lin L, Chan SO. Pertubation of CD44 function affects chi-asmatic routing of retinal axons in brain slice preparations of the mouse retinofugal pathway. Eur J Neurosci. 2003; 17:2299–312.27. Fischer AJ, Zelinka C, Scott MA. Heterogeneity of glia in the retina and optic nerve of birds and mammals. PLoS One. 2010; 5:1–15.
Article28. Kim JY, Sohn HJ, Lee EY, Goo YS, Kim DW, Seo JH. Expression of αB-crystallin in the peripapillary glial cells of the developing chick retina. Neurochem Res. 2011; 36:76–82.
Article29. Ghosh P, Mukherjee N, Ghosh K, Mallick S, Pal C, Las-kar A, et al. Prospective microglia and brain macrophage distribution pattern in normal rat brain shows age sensitive dispersal and stabilization with development. Indian J Exp Biol. 2015; 53:561–7.30. Greter M, Merad M. Regulation of microglia development and homeostasis. Glia. 2013; 61:121–7.
Article31. Yang H, Zheng S, Mao Y, Chen Z, Zheng C, Li H, et al. Modulating of ocular inflammation with macrophage migration inhibitory factor is associated with notch signaling in experimental autoimmune uveitis. Clin Exp Immunol. 2015; 183:280–93.32. Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014; 32:367–402.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between Expression of CD44, Bcl-2, PCNA, and Apoptosis in Neuroblastoma According to Shimada Histology
- Expression of CD44 Splicing Variants v4/5 and v6 in Gastric Adenocarcinoma and Its Relationship with Prognostic Factors
- Expression of CD44 in Preinvasive Disease and Invasive Cancer in Cervix
- Immunohistochemieal Study of Expression of nm23 and CD44 Protein in
- Characterization of the antigenic phenotype of alphaB-crystallin-expressing peripapillary glial cells in the developing chick retina