Ann Lab Med.
2016 Nov;36(6):561-572. 10.3343/alm.2016.36.6.561.
A Population-Based Genomic Study of Inherited Metabolic Diseases Detected Through Newborn Screening
- Affiliations
-
- 1Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences and Technology, Sungkyunkwan University, Seoul, Korea. kimjw@skku.edu
- 2Green Cross Laboratories, Yongin, Korea.
- 3Samsung Biomedical Research Institute, Samsung Medical Center, Seoul, Korea.
- 4Department of Laboratory Medicine & Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2373595
- DOI: http://doi.org/10.3343/alm.2016.36.6.561
Abstract
- BACKGROUND
A newborn screening (NBS) program has been utilized to detect asymptomatic newborns with inherited metabolic diseases (IMDs). There have been some bottlenecks such as false-positives and imprecision in the current NBS tests. To overcome these issues, we developed a multigene panel for IMD testing and investigated the utility of our integrated screening model in a routine NBS environment. We also evaluated the genetic epidemiologic characteristics of IMDs in a Korean population.
METHODS
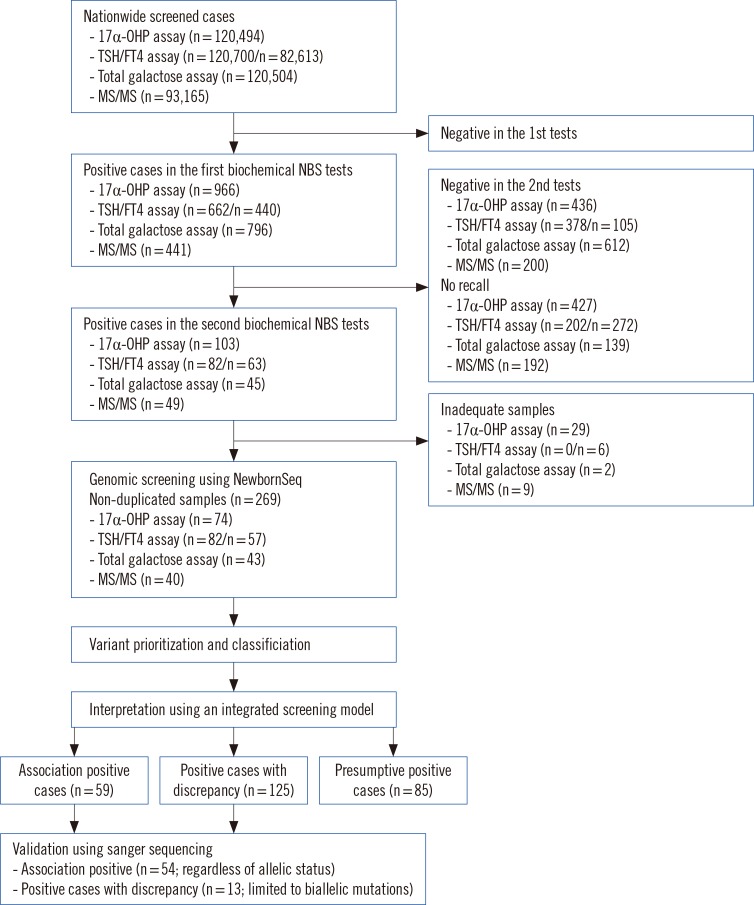
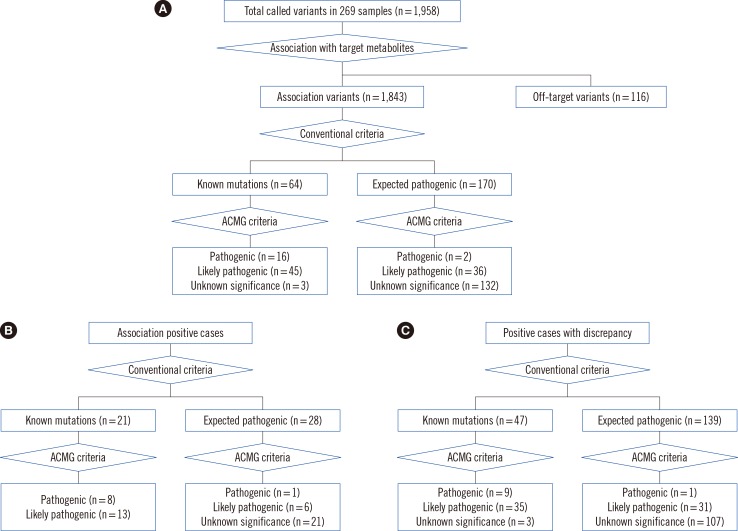
In total, 269 dried blood spots with positive results from current NBS tests were collected from 120,700 consecutive newborns. We screened 97 genes related to NBS in Korea and detected IMDs, using an integrated screening model based on biochemical tests and next-generation sequencing (NGS) called NewbornSeq. Haplotype analysis was conducted to detect founder effects.
RESULTS
The overall positive rate of IMDs was 20%. We identified 10 additional newborns with preventable IMDs that would not have been detected prior to the implementation of our NGS-based platform NewbornSeq. The incidence of IMDs was approximately 1 in 2,235 births. Haplotype analysis demonstrated founder effects in p.Y138X in DUOXA2, p.R885Q in DUOX2, p.Y439C in PCCB, p.R285Pfs*2 in SLC25A13, and p.R224Q in GALT.
CONCLUSIONS
Through a population-based study in the NBS environment, we highlight the screening and epidemiological implications of NGS. The integrated screening model will effectively contribute to public health by enabling faster and more accurate IMD detection through NBS. This study suggested founder mutations as an explanation for recurrent IMD-causing mutations in the Korean population.
Keyword
MeSH Terms
-
Computational Biology
DNA/chemistry/isolation & purification/metabolism
Dried Blood Spot Testing
Galactokinase
Genomics
Haplotypes
High-Throughput Nucleotide Sequencing
Humans
Incidence
Infant, Newborn
Membrane Proteins/genetics
Metabolic Diseases/*diagnosis/epidemiology/genetics
Metabolism, Inborn Errors/diagnosis/epidemiology/genetics
Mitochondrial Membrane Transport Proteins/genetics
Neonatal Screening
Polymorphism, Genetic
Republic of Korea/epidemiology
Sequence Analysis, DNA
DNA
Galactokinase
Membrane Proteins
Mitochondrial Membrane Transport Proteins
Figure
Reference
-
1. Feuchtbaum L, Carter J, Dowray S, Currier RJ, Lorey F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet Med. 2012; 14:937–945. PMID: 22766612.2. Yoon HR, Lee KR, Kang S, Lee DH, Yoo HW, Min WK, et al. Screening of newborns and high-risk group of children for inborn metabolic disorders using tandem mass spectrometry in South Korea: a three-year report. Clin Chim Acta. 2005; 354:167–180. PMID: 15748614.3. Zytkovicz TH, Fitzgerald EF, Marsden D, Larson CA, Shih VE, Johnson DM, et al. Tandem mass spectrometric analysis for amino, organic, and fatty acid disorders in newborn dried blood spots: a two-year summary from the New England Newborn Screening Program. Clin Chem. 2001; 47:1945–1955. PMID: 11673361.4. Wilcken B, Wiley V, Hammond J, Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003; 348:2304–2312. PMID: 12788994.5. Schulze A, Lindner M, Kohlmüller D, Olgemöller K, Mayatepek E, Hoffmann GF. Expanded newborn screening for inborn errors of metabolism by electrospray ionization-tandem mass spectrometry: results, outcome, and implications. Pediatrics. 2003; 111:1399–1406. PMID: 12777559.6. Mak CM, Lee HC, Chan AY, Lam CW. Inborn errors of metabolism and expanded newborn screening: review and update. Crit Rev Clin Lab Sci. 2013; 50:142–162. PMID: 24295058.7. Sahai I, Marsden D. Newborn screening. Crit Rev Clin Lab Sci. 2009; 46:55–82. PMID: 19255915.8. Park KJ, Kim JW. Perspectives on next-generation newborn screening. Lab Med Online. 2015; 5:169–175.9. Knoppers BM, Sénécal K, Borry P, Avard D. Whole-genome sequencing in newborn screening programs. Sci Transl Med. 2014; 6:229cm2.10. Bhattacharjee A, Sokolsky T, Wyman SK, Reese MG, Puffenberger E, Strauss K, et al. Development of DNA confirmatory and high-risk diagnostic testing for newborns using targeted next-generation DNA sequencing. Genet Med. 2015; 17:337–347. PMID: 25255367.11. Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012; 4:154ra135.12. Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. 2015; 3:377–387. PMID: 25937001.13. Fernández-Marmiesse A, Morey M, Pineda M, Eiris J, Couce ML, Castro-Gago M, et al. Assessment of a targeted resequencing assay as a support tool in the diagnosis of lysosomal storage disorders. Orphanet J Rare Dis. 2014; 9:59. PMID: 24767253.14. Cao YY, Qu YJ, Song F, Zhang T, Bai JL, Jin YW, et al. Fast clinical molecular diagnosis of hyperphenylalaninemia using next-generation sequencing-based on a custom AmpliSeq panel and Ion Torrent PGM sequencing. Mol Genet Metab. 2014; 113:261–266. PMID: 25456745.15. Calvo SE, Compton AG, Hershman SG, Lim SC, Lieber DS, Tucker EJ, et al. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci Transl Med. 2012; 4:118ra10.16. Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969-1996. Pediatrics. 2000; 105:e10. PMID: 10617747.17. Dionisi-Vici C, Rizzo C, Burlina AB, Caruso U, Sabetta G, Uziel G, et al. Inborn errors of metabolism in the Italian pediatric population: a national retrospective survey. J Pediatr. 2002; 140:321–327. PMID: 11953730.18. Niu DM, Chien YH, Chiang CC, Ho HC, Hwu WL, Kao SM, et al. Nationwide survey of extended newborn screening by tandem mass spectrometry in Taiwan. J Inherit Metab Dis. 2010; 33:S295–S305. PMID: 20567911.19. Narumi S, Muroya K, Asakura Y, Aachi M, Hasegawa T. Molecular basis of thyroid dyshormonogenesis: genetic screening in population-based Japanese patients. J Clin Endocrinol Metab. 2011; 96:E1838–E1842. PMID: 21900383.20. Narumi S, Muroya K, Asakura Y, Adachi M, Hasegawa T. Transcription factor mutations and congenital hypothyroidism: systematic genetic screening of a population-based cohort of Japanese patients. J Clin Endocrinol Metab. 2010; 95:1981–1985. PMID: 20157192.21. Narumi S, Muroya K, Abe Y, Yasui M, Asakura Y, Adachi M, et al. TSHR mutations as a cause of congenital hypothyroidism in Japan: a population-based genetic epidemiology study. J Clin Endocrinol Metab. 2009; 94:1317–1323. PMID: 19158199.22. Kalaydjieva L, Perez-Lezaun A, Angelicheva D, Onengut S, Dye D, Bosshard NU, et al. A founder mutation in the GK1 gene is responsible for galactokinase deficiency in Roma (Gypsies). Am J Hum Genet. 1999; 65:1299–1307. PMID: 10521295.23. Suzuki M, West C, Beutler E. Large-scale molecular screening for galactosemia alleles in a pan-ethnic population. Hum Genet. 2001; 109:210–215. PMID: 11511927.24. Lu YB, Kobayashi K, Ushikai M, Tabata A, Iijima M, Li MX, et al. Frequency and distribution in East Asia of 12 mutations identified in the SLC25A13 gene of Japanese patients with citrin deficiency. J Hum Genet. 2005; 50:338–346. PMID: 16059747.25. Chien YH, Chiang SC, Huang A, Chou SP, Tseng SS, Huang YT, et al. Mutation spectrum in Taiwanese patients with phenylalanine hydroxylase deficiency and a founder effect for the R241C mutation. Hum Mutat. 2004; 23:206. PMID: 14722928.26. Wang T, Okano Y, Eisensmith RC, Harvey ML, Lo WH, Huang SZ, et al. Founder effect of a prevalent phenylketonuria mutation in the Oriental population. Proc Natl Acad Sci U S A. 1991; 88:2146–2150. PMID: 2006152.27. Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010; 38:e164. PMID: 20601685.28. Liu X, Jian X, Boerwinkle E. dbNSFP v2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum Mutat. 2013; 34:E2393–E2402. PMID: 23843252.29. Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014; 42:D980–D985. PMID: 24234437.30. Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014; 133:1–9. PMID: 24077912.31. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. PMID: 25741868.32. Kim YU, Kim SH, Jin H, Park YK, Ji M, Kim YJ. The Korean HapMap Project website. Genomics Inform. 2008; 6:91–94.33. Satoh M, Aso K, Ogikubo S, Ogasawara A, Saji T. Genetic analysis in children with transient thyroid dysfunction or subclinical hypothyroidism detected on neonatal screening. Clin Pediatr Endocrinol. 2009; 18:95–100. PMID: 23926367.34. De Marco G, Agretti P, Montanelli L, Di Cosmo C, Bagattini B, De Servi M, et al. Identification and functional analysis of novel dual oxidase 2 (DUOX2) mutations in children with congenital or subclinical hypothyroidism. J Clin Endocrinol Metab. 2011; 96:E1335–E1339. PMID: 21565790.35. Coffey AJ, Durkie M, Hague S, McLay K, Emmerson J, Lo C, et al. A genetic study of Wilson's disease in the United Kingdom. Brain. 2013; 136:1476–1487. PMID: 23518715.36. Vockley J. Metabolism as a complex genetic trait, a systems biology approach: implications for inborn errors of metabolism and clinical diseases. J Inherit Metab Dis. 2008; 31:619–629. PMID: 18836848.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Seven-year experience with inherited metabolic disorders screening by tandem mass spectrometry
- Perspectives on Next-Generation Newborn Screening
- Screening newborns for metabolic disorders based on targeted metabolomics using tandem mass spectrometry
- The prevalence of pediatric endocrine and metabolic diseases in Korea
- A cost-benefit analysis on tandem mass spectrometry of inherited metabolic diseases in Korea