Ann Lab Med.
2016 May;36(3):223-229. 10.3343/alm.2016.36.3.223.
Plasma Levels and Diagnostic Utility of Macrophage Colony-Stimulating Factor, Matrix Metalloproteinase-9, and Tissue Inhibitor of Metalloproteinases-1 as New Biomarkers of Breast Cancer
- Affiliations
-
- 1Department of Biochemical Diagnostics, Medical University, Bialystok, Poland. zdb@umb.edu.pl
- 2Laboratory of Esthetic Medicine, Medical University, Bialystok, Poland.
- 3Department of HaematologicalDiagnostics, Medical University, Bialystok, Poland.
- KMID: 2373531
- DOI: http://doi.org/10.3343/alm.2016.36.3.223
Abstract
- BACKGROUND
Macrophage colony-stimulating factor (M-CSF), matrix metalloproteinase-9 (MMP-9), and its specific tissue inhibitor - tissue inhibitor of metalloproteinases-1 (TIMP-1) may play an important role in the pathogenesis and spread of cancer. We investigated the plasma levels of M-CSF, MMP-9, and TIMP-1 in comparison with a commonly accepted tumor marker CA 15-3 in breast cancer patients and in control groups.
METHODS
The cohort included 110 breast cancer patients in groups at stages I-IV. The control group consisted of 50 healthy volunteers and 50 benign tumor patients. Plasma levels of M-CSF, MMP-9, and TIMP-1 were determined by using ELISA, while CA 15-3 concentrations were determined by using chemiluminescent microparticle immunoassay (CMIA).
RESULTS
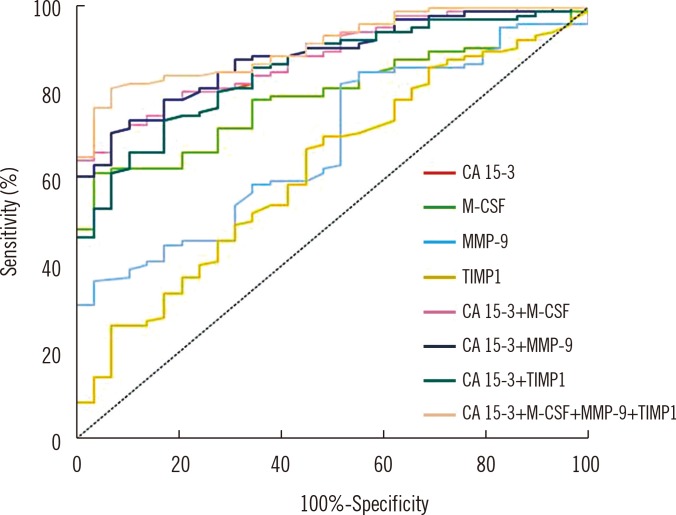
The results showed significant differences in concentrations of the analyzed parameters and in levels of CA 15-3 between the groups of breast cancer patients and the two control groups. Diagnosis using these markers was equal to that using CA 15-3 in terms of sensitivity, predictive values of positive and negativetest results (PPV, NPV) and area under the ROC curve (AUC) in the studied groups. The diagnostic specificities of MMP-9, TIMP-1, M-CSF, and CA 15-3 showed equally high values (95%). The combined use of all tested parameters with CA 15-3 resulted in increased sensitivity, NPV, and AUC, especially in the combination of M-CSF with tumor markers (76%, 64%, and 0.8653).
CONCLUSIONS
These findings suggest the tested parameters are useful in the diagnosis of breast cancer patients (except stage I), when combined with CA 15-3.
Keyword
MeSH Terms
-
Adult
Aged
Area Under Curve
Biomarkers, Tumor/*blood
Breast Neoplasms/*diagnosis/genetics/pathology
Case-Control Studies
Female
Humans
Macrophage Colony-Stimulating Factor/*blood
Matrix Metalloproteinase 9/*blood
Middle Aged
Mucin-1/blood
Neoplasm Staging
Poland
ROC Curve
Sensitivity and Specificity
Tissue Inhibitor of Metalloproteinase-1/*blood
Biomarkers, Tumor
Macrophage Colony-Stimulating Factor
Matrix Metalloproteinase 9
Mucin-1
Tissue Inhibitor of Metalloproteinase-1
Figure
Reference
-
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society;2014. p. 4.2. Trichopoulos D, Adami HO, Ekbom A, Hsieh CC, Lagiou P. Early life events and conditions and breast cancer risk: from epidemiology to etiology. Int J Cancer. 2008; 122:481–485. PMID: 18022897.
Article3. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007; 25:5287–5312. PMID: 17954709.
Article4. Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci. 2006; 11:479–491. PMID: 16146745.
Article5. Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004; 10:7621–7628. PMID: 15569994.
Article6. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000; 1477:267–283. PMID: 10708863.
Article7. Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1,-2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998; 101:1478–1487. PMID: 9502791.8. Talvensaari-Mattila A, Turpeenniemi-Hujanen T. High preoperative serum TIMP-1 is a prognostic indicator for survival in breast carcinoma. Breast Cancer Res Treat. 2005; 89:29–34. PMID: 15666194.
Article9. Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002; 7:147–162. PMID: 12465600.10. Sobin LH, Gospodarowicz MK, editors. TNM classification of malignant tumours. 7th ed. Chichester: John Wiley & Sons;2011. p. 181–183.11. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003; 53:5–26. PMID: 12568441.
Article12. Ławicki S, Będkowska GE, Gacuta-Szumarska E, Knapp P, Szmitkowski M. Pretreatment plasma levels and diagnostic utility of hematopoietic cytokines in cervical cancer or cervical intraepithelial neoplasia patients. Folia Histochem Cytobiol. 2012; 50:213–219. PMID: 22763962.
Article13. Ławicki S, Będkowska GE, Gacuta-Szumarska E, Czygier M, Szmitkowski M. The plasma levels and diagnostics utility of selected hematopoietic growth factors in endometrial cancer patients and with myoma uteri. Pol Merkur Lekarski. 2010; 28:354–358. PMID: 20568396.14. Ławicki S, Gacuta-Szumarska E, Będkowska GE, Szmitkowski M. Hematopoietic cytokines as tumor markers in gynecological malignancies. A multivariate analysis in epithelial ovarian cancer patients. Growth Factors. 2012; 30:357–366. PMID: 22988839.
Article15. Czygier M, Ławicki S, Szmitkowski M. The plasma level of sL-selectin, myeloperoxidase (MPO) and granulocyte-colony stimulating factor (G-CSF) in breast cancer patients after surgery. Przegl Lek. 2009; 66:433–436. PMID: 20043590.16. Vasiliades G, Kopanakis N, Vasiloglou M, Zografos G, Margaris H, Masselou K, et al. Role of the hematopoietic cytokines SCF, IL-3, GM-CSF and M-CSF in the diagnosis of pancreatic and ampullary cancer. Int J Biol Markers. 2012; 27:e186–e194. PMID: 22865301.
Article17. Bahar B, AcedilAyc Iota B, Çoşkun U, Büyükberber S, Benekli M, Yildiz R. Granulocyte colony stimulating factor (G-CSF) and macrophage colony stimulating factor (M-CSF) as potential tumor markers in non small cell lung cancer diagnosis. Asian Pac J Cancer Prev. 2010; 11:709–712. PMID: 21039040.18. McDermott RS, Deneux L, Mosseri V, Védrenne J, Clough K, Fourquet A, et al. Circulating macrophage colony stimulating factor as a marker of tumour progression. Eur Cytokine Netw. 2002; 13:121–127. PMID: 11956031.19. Kaciński BM. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med. 1995; 27:79–85. PMID: 7742005.20. Scholl SM, Lidereau R, de la Rochefordière A, Le-Nir CC, Mosseri V, Noguès C, et al. Circulating levels of the macrophage-colony stimulating factor CSF-1 in primary and metastatic breast cancer patients. A pilot study. Breast Cancer Res Treat. 1996; 39:275–283. PMID: 8877007.21. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003; 92:827–839. PMID: 12730128.22. Giannelli G, Erriquez R, Fransvea E, Daniele A, Trerotoli P, Schittulli F, et al. Proteolytic imbalance is reversed after therapeutic surgery in breast cancer patients. Int J Cancer. 2004; 109:782–785. PMID: 14999790.
Article23. Jinga DC, Blidaru A, Condrea I, Ardeleanu C, Dragomir C, Szegli G, et al. MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast cancer: correlations with prognostic factors. J Cell Mol Med. 2006; 10:499–510. PMID: 16796815.
Article24. Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, et al. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer. 2008; 122:2050–2056. PMID: 18172859.
Article25. Voorzanger-Rousselot N, Juillet F, Mareau E, Zimmermann J, Kalebic T, Garnero P. Association of 12 serum biochemical markers of angiogenesis, tumour invasion and bone turnover with bone metastases from breast cancer: a crossectional and longitudinal evaluation. Br J Cancer. 2006; 95:506–514. PMID: 16880790.
Article26. Ławicki S, Bedkowska GE, Szmitkowski M. VEGF, M-CSF and CA 15-3 as a new tumor marker panel in breast malignancies: a multivariate analysis with ROC curve. Growth Factors. 2013; 31:98–105. PMID: 23688065.
Article27. Ławicki S, Szmitkowski M, Wojtukiewicz M. The pretreatment plasma level and diagnostic utility of M-CSF in benign breast tumor and breast cancer patients. Clin Chim Acta. 2006; 371:112–116. PMID: 16631152.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Matrix Metalloproteinases and Its Inhibitor in Gastric Adenocarcinoma
- Clinical significance of the serum levels of matrix metalloproteinases and the tissue inhibitors of metalloproteinases for gastric and colorectal cancer patients
- Matrix Metalloproteinases and Their Inhibitors in Gastric Carcinoma
- The effect of periodontal flap surgery on Matrix metalloproteinases (MMPs) and Tissue inhibitors of matrix metalloproteinase-1 (TIMP-1) levels in gingival crevicular fluids of periodontitis patients
- The Effect of Granulocyte Colony Stimulating Factor and Granulocyte Macrophage Colony Stimulating Factor on Expression of Matrix Metalloproteinase-2, 9 in Mouse Embryos