Korean J Ophthalmol.
2017 Apr;31(2):95-101. 10.3341/kjo.2017.31.2.95.
Natural Short-term Course of Recurrent Macular Edema Following Intravitreal Bevacizumab Therapy in Branch Retinal Vein Occlusion
- Affiliations
-
- 1Department of Ophthalmology, Kim's Eye Hospital, Konyang University College of Medicine, Seoul, Korea. kimoph@gmail.com
- KMID: 2373464
- DOI: http://doi.org/10.3341/kjo.2017.31.2.95
Abstract
- PURPOSE
To evaluate the 3-month natural course of recurrent macular edema secondary to branch retinal vein occlusion (BRVO) treated with intravitreal bevacizumab.
METHODS
This retrospective, observational study included 36 eyes with macular edema secondary to BRVO. All patients were initially treated with intravitreal bevacizumab for macular edema. Recurrence of macular edema was either not treated (untreated group) or treated with a single intravitreal bevacizumab injection (treated group). Central foveal thickness (CFT) and best-corrected visual acuity (BCVA) were compared at the time of recurrence and 3 months later.
RESULTS
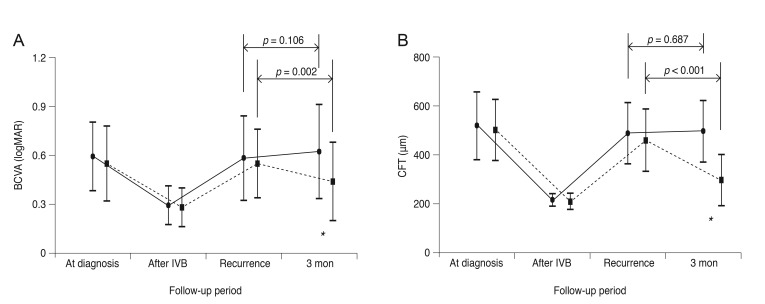
At the time of recurrence, the mean CFT and logarithm of the minimum angle of resolution BCVA were 484.9 ± 124.1 µm and 0.58 ± 0.26 in the untreated group (n = 19) and 456.3 ± 126.8 µm and 0.51 ± 0.21 in the treated group (n = 17), respectively. Three months later, the mean CFT and BCVA had changed to 493.7 ± 123.9 µm and 0.62 ± 0.29 in the untreated group and 294.7 ± 104.4 µm and 0.40 ± 0.24 in the treated group, respectively. The differences in CFT and BCVA between the two time points were not significant in the untreated group (p = 0.106 and p = 0.687, respectively), whereas statistically significant differences were noted in the treated group (p = 0.002 and p < 0.001, respectively).
CONCLUSIONS
Unlike the first episode of macular edema following BRVO, recurrent macular edema following intravitreal bevacizumab therapy did not spontaneously resolve, suggesting the potential benefit of prompt treatment.
MeSH Terms
Figure
Reference
-
1. Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010; 117:1094–1101.e5. PMID: 20430447.
Article2. Argon laser photocoagulation for macular edema in branch vein occlusion: the Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984; 98:271–282. PMID: 6383055.3. Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res. 2014; 41:1–25. PMID: 24769221.
Article4. Ramezani A, Entezari M, Moradian S, et al. Intravitreal triamcinolone for acute branch retinal vein occlusion: a randomized clinical trial. J Ophthalmic Vis Res. 2011; 6:101–108. PMID: 22454718.5. Shroff D, Mehta DK, Arora R, et al. Natural history of macular status in recent-onset branch retinal vein occlusion: an optical coherence tomography study. Int Ophthalmol. 2008; 28:261–268. PMID: 17668149.
Article6. Moradian S, Faghihi H, Sadeghi B, et al. Intravitreal bevacizumab vs. sham treatment in acute branch retinal vein occlusion with macular edema: results at 3 months (report 1). Graefes Arch Clin Exp Ophthalmol. 2011; 249:193–200. PMID: 21337043.7. Kwon SI, Kim YW, Bang YW, et al. Comparison of natural course, intravitreal triamcinolone, and intravitreal bevacizumab for treatment of macular edema secondary to branch retinal vein occlusion. J Ocul Pharmacol Ther. 2013; 29:5–9. PMID: 23237542.
Article8. Tan MH, McAllister IL, Gillies ME, et al. Randomized controlled trial of intravitreal ranibizumab versus standard grid laser for macular edema following branch retinal vein occlusion. Am J Ophthalmol. 2014; 157:237–247. PMID: 24112635.
Article9. Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011; 118:1594–1602. PMID: 21684606.
Article10. Gunduz K, Bakri SJ. Intravitreal bevacizumab for macular oedema secondary to branch retinal vein occlusion. Eye (Lond). 2008; 22:1168–1171. PMID: 18344969.11. Hanada N, Iijima H, Sakurada Y, Imasawa M. Recurrence of macular edema associated with branch retinal vein occlusion after intravitreal bevacizumab. Jpn J Ophthalmol. 2012; 56:165–174. PMID: 22183139.
Article12. Zhu D, Jin ZY, Tao Y, Jonas JB. Meta-analysis of the effect of intravitreal bevacizumab in branch retinal vein occlusion. J Ocul Pharmacol Ther. 2013; 29:523–529. PMID: 23537150.
Article13. Kim JY, Park SP. Comparison between intravitreal bevacizumab and triamcinolone for macular edema secondary to branch retinal vein occlusion. Korean J Ophthalmol. 2009; 23:259–265. PMID: 20046685.
Article14. Lee K, Jung H, Sohn J. Comparison of injection of intravitreal drugs with standard care in macular edema secondary to branch retinal vein occlusion. Korean J Ophthalmol. 2014; 28:19–25. PMID: 24505197.
Article15. Yasuda S, Kondo M, Kachi S, et al. Rebound of macular edema after intravitreal bevacizumab therapy in eyes with macular edema secondary to branch retinal vein occlusion. Retina. 2011; 31:1075–1082. PMID: 21478810.
Article16. Matsumoto Y, Freund KB, Peiretti E, et al. Rebound macular edema following bevacizumab (Avastin) therapy for retinal venous occlusive disease. Retina. 2007; 27:426–431. PMID: 17420693.
Article17. Jaissle GB, Szurman P, Feltgen N, et al. Predictive factors for functional improvement after intravitreal bevacizumab therapy for macular edema due to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2011; 249:183–192. PMID: 21337042.
Article18. Yunoki T, Miyakoshi A, Nakamura T, et al. Treatment of macular edema due to branch retinal vein occlusion with single or multiple intravitreal injections of bevacizumab. Jpn J Ophthalmol. 2012; 56:159–164. PMID: 22246387.
Article19. Yunoki T, Mitarai K, Yanagisawa S, et al. Effects of vitrectomy on recurrent macular edema due to branch retinal vein occlusion after intravitreal injection of bevacizumab. J Ophthalmol. 2013; 2013:415974. PMID: 23533707.
Article20. Ogino K, Tsujikawa A, Murakami T, et al. Grid photocoagulation combined with intravitreal bevacizumab for recurrent macular edema associated with retinal vein occlusion. Clin Ophthalmol. 2011; 5:1031–1036. PMID: 21845030.21. Chen SD, Sundaram V, Lochhead J, Patel CK. Intravitreal triamcinolone for the treatment of ischemic macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2006; 141:876–883. PMID: 16527226.
Article22. Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008; 146:508–512. PMID: 18635152.
Article23. Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology. 2013; 120:795–802. PMID: 23260261.
Article24. Terui T, Kondo M, Sugita T, et al. Changes in areas of capillary nonperfusion after intravitreal injection of bevacizumab in eyes with branch retinal vein occlusion. Retina. 2011; 31:1068–1074. PMID: 21451440.
Article25. Karagiannis DA, Karampelas MD, Soumplis VM, et al. Recurrence of macular edema in retinal vein occlusions after treatment with intravitreal ranibizumab (Lucentis). Can J Ophthalmol. 2011; 46:486–490. PMID: 22153634.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Short-term Effects of Intravitreal Bevacizumab Injection and Macular Edema Patterns in Branch Retinal Vein Occlusion
- The Efficacy of Intravitreal Bevacizumab in the Treatment of Macular Edema
- Short-term Effectiveness of Intravitreal Triamcinolone Injection for Refractory Macular Edema Secondary to Branch Retinal Vein Occlusion
- Effects of Intravitreal Bevacizumab Injection in 3 Types of Macular Edema Secondary to Branch Retinal Vein Occlusion
- Two Cases of Macular Edema Associated with Extramacular Branch Retinal Vein Occlusion