Korean J Gastroenterol.
2015 Mar;65(3):159-164. 10.4166/kjg.2015.65.3.159.
Ideal Vaccination Strategy in Inflammatory Bowel Disease
- Affiliations
-
- 1Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea. cklee92@paran.com
- KMID: 2373201
- DOI: http://doi.org/10.4166/kjg.2015.65.3.159
Abstract
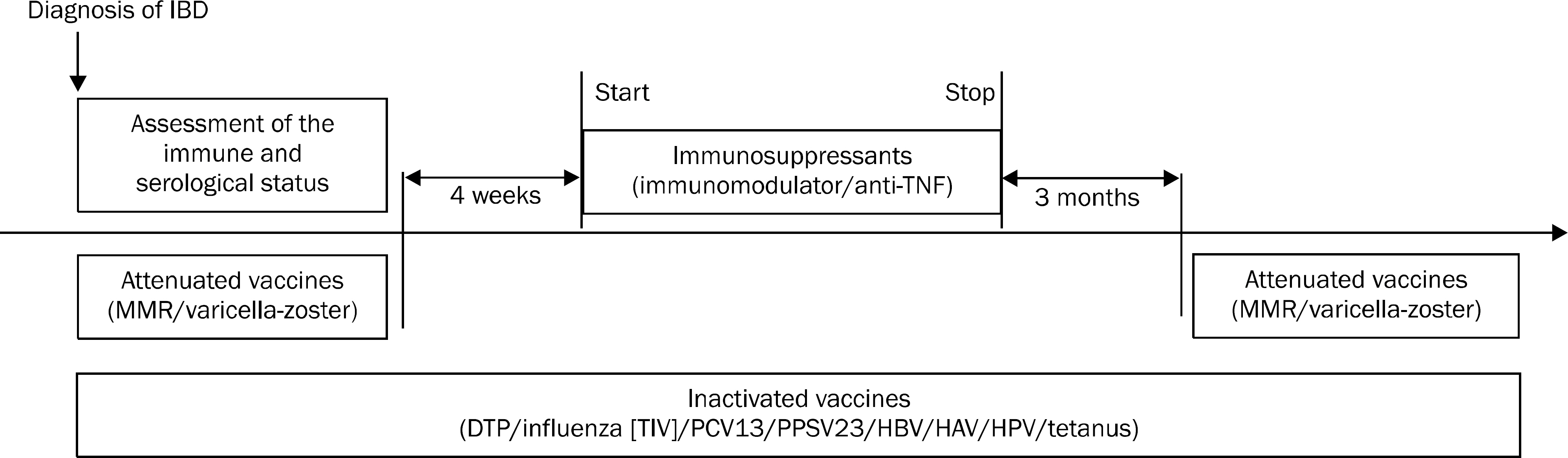
- Inflammatory bowel disease (IBD) is a long-standing disease that often requires long-term use of immunosuppressive agents including immunomodulators (such as azathioprine, 6-mercaptopurine and methotrexate) and tumor necrosis factor-alpha inhibitors (such as infliximab and adalimumab). Introduction of immunosuppressive therapies, however, involves the risk of host susceptibility to opportunistic infections in this patient population. Therefore, adequate immunization for vaccine-preventable infectious diseases is currently recommended for all patients with IBD and is emerging as an important target for quality improvements in IBD care. However, ongoing issues regarding underuse of immunization, safety and efficacy of vaccines in patients with IBD remain. For quality improvements in IBD care, all physicians should follow the recent immunization guidelines proposed by professional IBD societies. Additionally, there are ongoing needs for intensive educational programs regarding a role of immunization in long-term care of IBD and up-to-date immunization guidelines. Immunization status should be checked at the time of diagnosis of IBD and timely vaccination before initiation of immunosuppressive therapies can be a practical solution for maximizing the efficacy of vaccination at this point. Inactivated vaccines can be used safely irrespective of immunization status of patients, while attenuated vaccines are contraindicated in patients on immunosuppressive therapies. This article reviews an ideal strategy for vaccinating patients with IBD based on the currently recommended immunization guidelines.
Keyword
MeSH Terms
-
Antibodies, Monoclonal/therapeutic use
Humans
Immunosuppressive Agents/therapeutic use
Inflammatory Bowel Diseases/diagnosis/drug therapy/*immunology
Influenza Vaccines/immunology
Influenza, Human/prevention & control
Pneumonia/prevention & control
*Vaccination
Vaccines, Synthetic/immunology
Antibodies, Monoclonal
Immunosuppressive Agents
Influenza Vaccines
Vaccines, Synthetic
Figure
Reference
-
References
1. Dignass A, Van Assche G, Lindsay JO, et al. European Crohn's and Colitis Organisation (ECCO). The second European evi-dence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010; 4:28–62.
Article2. Lichtenstein GR, Hanauer SB, Sandborn WJ. Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009; 104:465–483.
Article3. D'Haens G, Baert F, van Assche G, et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open rand-omised trial. Lancet. 2008; 371:660–667.4. Colombel JF, Sandborn WJ, Reinisch W, et al. SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395.
Article5. Ramadas AV, Gunesh S, Thomas GA, Williams GT, Hawthorne AB. Natural history of Crohn's disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010; 59:1200–1206.
Article6. Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppre-ssants in Crohn's disease on the need for intestinal surgery. Gut. 2005; 54:237–241.
Article7. Toruner M, Loftus EV Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008; 134:929–936.
Article8. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT TM registry. Am J Gastroenterol. 2012; 107:1409–1422.9. Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013; 108:1268–1276.
Article10. Sands BE, Cuffari C, Katz J, et al. Guidelines for immunizations in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004; 10:677–692.
Article11. Melmed GY. Vaccination strategies for patients with inflammatory bowel disease on immunomodulators and biologics. Inflamm Bowel Dis. 2009; 15:1410–1416.
Article12. AGA quality and outcomes measures. [Internet]. Bethesda (MD): American Gastroenterological Association [updated 2012 Jun; cited 2015 Mar 9]. Available from:. http://www.gastro.org/practice/quality-initiatives/performance-measures/aga-quality-and-outcomes-measures.13. Melmed GY, Siegel CA. Quality improvement in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013; 9:286–292.14. Melmed GY, Siegel CA, Spiegel BM, et al. Quality indicators for inflammatory bowel disease: development of process and outcome measures. Inflamm Bowel Dis. 2013; 19:662–668.15. Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006; 101:1834–1840.
Article16. Wasan SK, Coukos JA, Farraye FA. Vaccinating the inflammatory bowel disease patient: deficiencies in gastroenterologists knowledge. Inflamm Bowel Dis. 2011; 17:2536–2540.
Article17. Yeung JH, Goodman KJ, Fedorak RN. Inadequate knowledge of immunization guidelines: a missed opportunity for preventing infection in immunocompromised IBD patients. Inflamm Bowel Dis. 2012; 18:34–40.
Article18. Yun HS, Min YW, Chang DK, et al. Factors associated with vaccination among inflammatory bowel disease patients in Korea. Korean J Gastroenterol. 2013; 61:203–208.
Article19. Kim SB, Park SJ, Chung SH, et al. Vaccination and comple-mentary and alternative medicine in patients with inflammatory bowel disease. Intest Res. 2014; 12:124–130.
Article20. Jung YS, Park JH, Kim HJ, et al. Insufficient knowledge of korean gastroenterologists regarding the vaccination of patients with inflammatory bowel disease. Gut Liver. 2014; 8:242–247.
Article21. Carrera E, Manzano R, Garrido E. Efficacy of the vaccination in inflammatory bowel disease. World J Gastroenterol. 2013; 19:1349–1353.
Article22. Rahier JF, Papay P, Salleron J, et al. European Crohn's and Colitis Organisation (ECCO). H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011; 60:456–462.
Article23. Cullen G, Bader C, Korzenik JR, Sands BE. Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut. 2012; 61:385–391.
Article24. Advisory Committee on Immunization Practices. Recommended adult immunization schedule: United States, 2013∗. Ann Intern Med. 2013; 158:191–199.25. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014; 58:309–318.
Article26. Dezfoli S, Melmed GY. Vaccination issues in patients with inflammatory bowel disease receiving immunosuppression. Gastroenterol Hepatol (N Y). 2012; 8:504–512.27. Melmed GY, Agarwal N, Frenck RW, et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010; 105:148–154.
Article28. Fiorino G, Peyrin-Biroulet L, Naccarato P, et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2012; 18:1042–1047.
Article29. Lee CK, Kim HS, Ye BD, et al. Korean Association for the Study of Intestinal Diseases (KASID) Study. Patients with Crohn's disease on anti-tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J Crohns Colitis. 2014; 8:384–391.
Article30. Abdallah J, Anna K, Hassan T, Jain A. Vaccination outcomes in inflammatory bowel disease (abstract). Gastroenterology. 2014; 146(Suppl 1):S170.31. Recommended immunization schedule for adults in Korea, by the Korean Society of Infectious Diseases, 2012. Clin Exp Vaccine Res. 2014; 3:110–112.32. Park SH, Yang SK, Park SK, et al. Efficacy of hepatitis A vaccination and factors impacting on seroconversion in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014; 20:69–74.
Article33. Kane S, Khatibi B, Reddy D. Higher incidence of abnormal Pap smears in women with inflammatory bowel disease. Am J Gastroenterol. 2008; 103:631–636.
Article34. Singh H, Demers AA, Nugent Z, Mahmud SM, Kliewer EV, Bernstein CN. Risk of cervical abnormalities in women with inflammatory bowel disease: a population-based nested case-control study. Gastroenterology. 2009; 136:451–458.
Article35. Lees CW, Critchley J, Chee N, et al. Lack of association between cervical dysplasia and IBD: a large case-control study. Inflamm Bowel Dis. 2009; 15:1621–1629.
Article36. Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008; 57:1–30.37. Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006; 4:1483–1490.
Article38. Cheent K, Nolan J, Shariq S, Kiho L, Pal A, Arnold J. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn's disease. J Crohns Colitis. 2010; 4:603–605.
Article39. Heller MM, Wu JJ, Murase JE. Fatal case of disseminated BCG infection after vaccination of an infant with in utero exposure to infliximab. J Am Acad Dermatol. 2011; 65:870.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vaccination strategies for Korean patients with inflammatory bowel disease
- How Should We Do Different Approach to Treat Inflammatory Bowel Disease by Gender Difference?
- Vaccination and Complementary and Alternative Medicine in Patients with Inflammatory Bowel Disease
- Response to hepatitis B vaccination in patients with inflammatory bowel disease: a prospective observational study in Korea
- Artificial intelligence in inflammatory bowel disease: implications for clinical practice and future directions