Intest Res.
2018 Oct;16(4):599-608. 10.5217/ir.2018.00012.
Response to hepatitis B vaccination in patients with inflammatory bowel disease: a prospective observational study in Korea
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 2434162
- DOI: http://doi.org/10.5217/ir.2018.00012
Abstract
- BACKGROUND/AIMS
Testing for hepatitis B virus (HBV) serologic markers and appropriate vaccination are required in the management of inflammatory bowel disease (IBD) patients. We evaluated immunogenicity for HBV in IBD patients and the response to the HBV vaccination.
METHODS
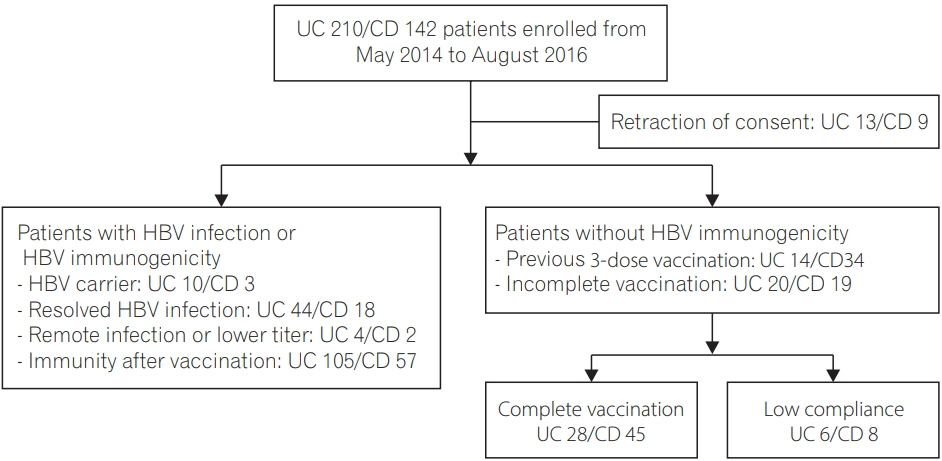
From May 2014 to August 2016, patients diagnosed with IBD were prospectively included and evaluated for antibody to hepatitis B surface antigen, antibody to hepatitis B core antigen, and antibody to hepatitis B surface antigen. Among the 73 patients who were confirmed with nonimmunity to HBV, 44 patients who had completed the 3-dose HBV vaccination series received a single booster vaccination, while 29 patients who had not completed the vaccinations series or were unsure of receiving the vaccination received a full vaccination series.
RESULTS
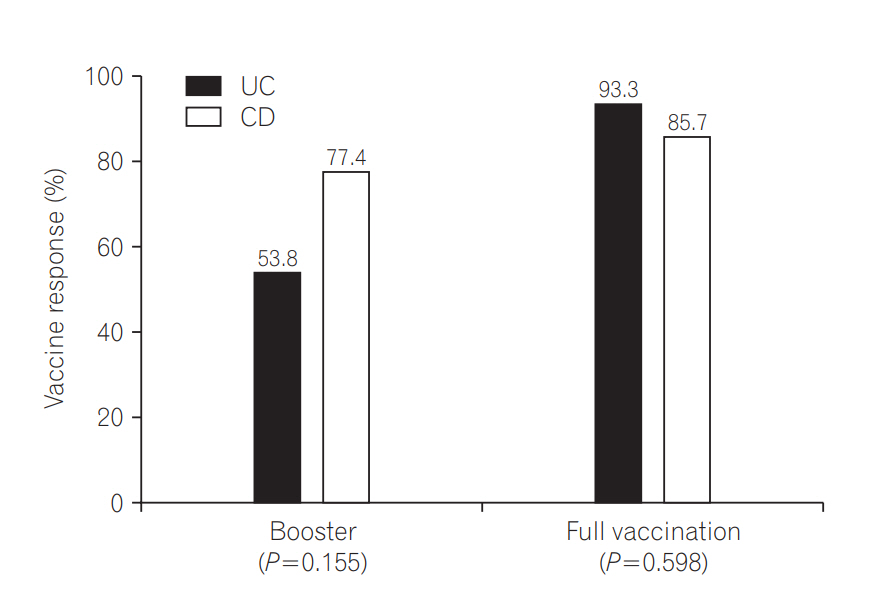
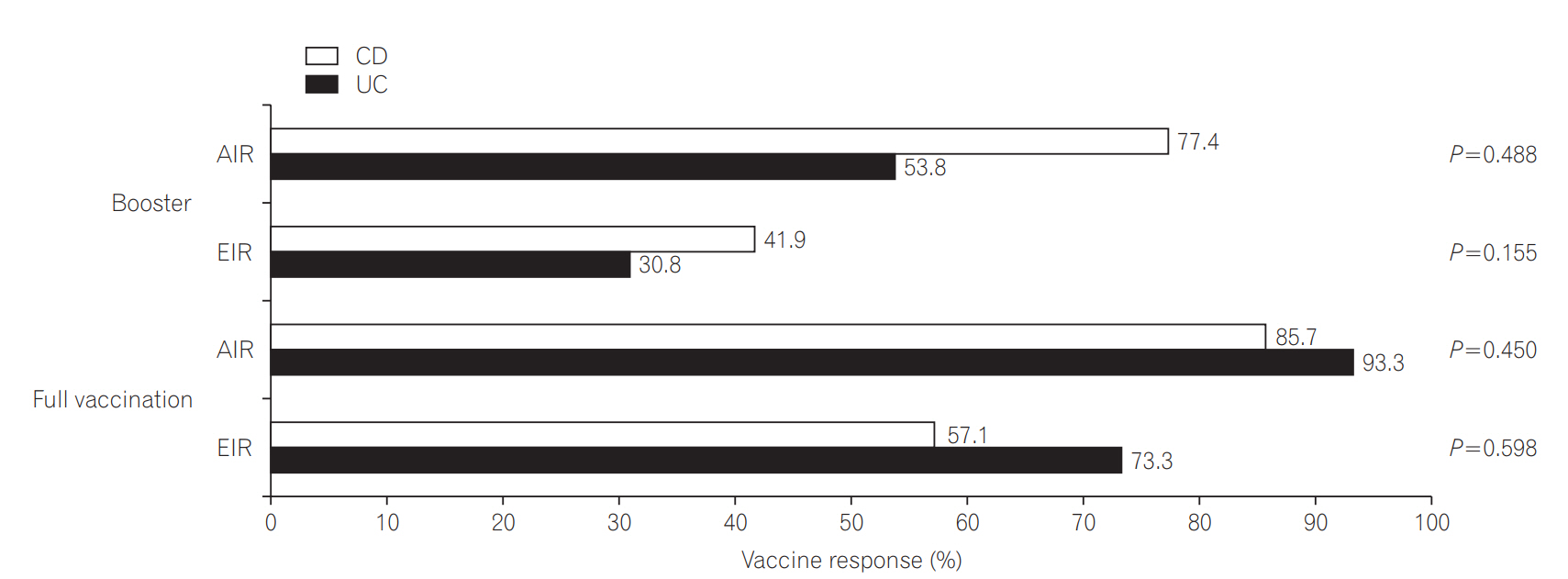
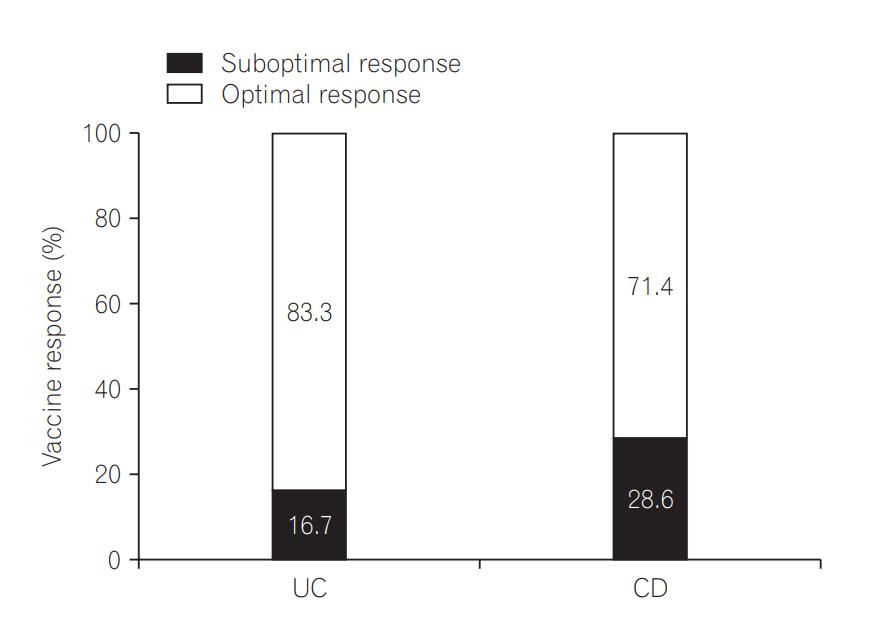
An optimal response was obtained in 70.5% of the patients in the booster group, and 89.7% of the patients in the full vaccination group. Age younger than 26 years (odds ratio [OR], 6.01; 95% confidence interval [CI], 1.15-31.32; P=0.033) and a complete previous vaccination series (OR, 0.15; 95% CI, 0.03-0.80; P=0.026) were associated with optimal vaccine response. Previous complete vaccination series (OR, 0.11; 95% CI, 0.02-0.73; P=0.022) was the only predictive factor for lower compliance.
CONCLUSIONS
The response to the HBV vaccination was lower in patients older than 26 years and for those patients with a complete vaccination history. Since patients with a complete vaccination history also had poor compliance, serum HBV-titers should be checked more thoroughly, and a full vaccination series should be administered in cases when there is a negative response to the booster vaccination.
MeSH Terms
Figure
Cited by 1 articles
-
Efficacy of hepatitis B vaccination in patients with ulcerative colitis: a prospective cohort study
Anurag Mishra, Amarender Singh Puri, Sanjeev Sachdeva, Ashok Dalal
Intest Res. 2022;20(4):445-451. doi: 10.5217/ir.2021.00106.
Reference
-
1. Altunöz ME, Senateş E, Yeşil A, Calhan T, Ovünç AO. Patients with inflammatory bowel disease have a lower response rate to HBV vaccination compared to controls. Dig Dis Sci. 2012; 57:1039–1044.
Article2. Huang ML, Xu XT, Shen J, Qiao YQ, Dai ZH, Ran ZH. Prevalence and factors related to hepatitis B and C infection in inflammatory bowel disease patients in China: a retrospective study. J Crohns Colitis. 2014; 8:282–287.
Article3. Gisbert JP, Chaparro M, Esteve M. Review article: prevention and management of hepatitis B and C infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011; 33:619–633.
Article4. Bernal I, Domènech E, García-Planella E, Cabré E, Gassull MA. Opportunistic infections in patients with inflammatory bowel disease undergoing immunosuppressive therapy. Gastroenterol Hepatol. 2003; 26:19–22.5. Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset still’s disease. J Rheumatol. 2003; 30:1624–1625.6. Wei SC, Chang TA, Chao TH, et al. Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease. Intest Res. 2017; 15:266–284.
Article7. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017; 112:241–258.
Article8. Moses J, Alkhouri N, Shannon A, et al. Hepatitis B immunity and response to booster vaccination in children with inflammatory bowel disease treated with infliximab. Am J Gastroenterol. 2012; 107:133–138.
Article9. Kim H, Shin AR, Chung HH, et al. Recent trends in hepatitis B virus infection in the general Korean population. Korean J Intern Med. 2013; 28:413–419.
Article10. Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002; 17:457–462.
Article11. Lee H, Lee H, Cho Y, Oh K, Ki M. Changes in seroprevalence of hepatitis B surface antigen and epidemiologic characteristics in the Republic of Korea, 1998-2013. Epidemiol Health. 2015; 37:e2015055. doi: 10.4178/epih/e2015055.12. Cho YK, Song BC. Prevention of viral hepatitis and vaccination. Korean J Med. 2012; 82:123–133.
Article13. López-Serrano P, Pérez-Calle JL, Sánchez-Tembleque MD. Hepatitis B and inflammatory bowel disease: role of antiviral prophylaxis. World J Gastroenterol. 2013; 19:1342–1348.
Article14. Ben Musa R, Gampa A, Basu S, et al. Hepatitis B vaccination in patients with inflammatory bowel disease. World J Gastroenterol. 2014; 20:15358–15366.
Article15. Lee YJ, Cheon JH, Kim JH, et al. Clinical efficacy of beclomethasone dipropionate in Korean patients with ulcerative colitis. Yonsei Med J. 2017; 58:144–149.
Article16. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002; 122:512–530.
Article17. Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology. 2010; 53:20–28.
Article18. Kim ES, Cho KB, Park KS, et al. Prevalence of hepatitis-B viral markers in patients with inflammatory bowel disease in a hepatitis-B-endemic area: inadequate protective antibody levels in young patients. J Clin Gastroenterol. 2014; 48:553–558.
Article19. Yun HS, Min YW, Chang DK, et al. Factors associated with vaccination among inflammatory bowel disease patients in Korea. Korean J Gastroenterol. 2013; 61:203–208.
Article20. Coates T, Wilson R, Patrick G, André F, Watson V. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther. 2001; 23:392–403.
Article21. Poorolajal J, Hooshmand E. Booster dose vaccination for preventing hepatitis B. Cochrane Database Syst Rev. 2016; (6):CD008256. doi: 10.1002/14651858.CD008256.pub3.
Article22. Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011; 53:68–75.
Article23. Gisbert JP, Chaparro M. Vaccination strategies in patients with IBD. Nat Rev Gastroenterol Hepatol. 2013; 10:277–285.
Article24. Bruce MG, Bruden D, Hurlburt D, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016; 214:16–22.
Article25. Cossio-Gil Y, Martínez-Gómez X, Campins-Martí M, et al. Immunogenicity of hepatitis B vaccine in patients with inflammatory bowel disease and the benefits of revaccination. J Gastroenterol Hepatol. 2015; 30:92–98.
Article26. Fisman DN, Agrawal D, Leder K. The effect of age on immunologic response to recombinant hepatitis B vaccine: a metaanalysis. Clin Infect Dis. 2002; 35:1368–1375.
Article27. Vida Pérez L, Gómez Camacho F, García Sánchez V, et al. Adequate rate of response to hepatitis B virus vaccination in patients with inflammatory bowel disease. Med Clin (Barc). 2009; 132:331–335.28. Fattal-German M. Immunocompetence in the elderly. Ann Pharm Fr. 1992; 50:13–24.29. Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines. 2008; 7:467–479.
Article30. Kim ES. Inflammatory bowel disease is no longer a risk factor of viral hepatitis infection in Asia. Intest Res. 2017; 15:5–6.
Article31. Walayat S, Ahmed Z, Martin D, Puli S, Cashman M, Dhillon S. Recent advances in vaccination of non-responders to standard dose hepatitis B virus vaccine. World J Hepatol. 2015; 7:2503–2509.
Article32. Agarwal N, Ollington K, Kaneshiro M, Frenck R, Melmed GY. Are immunosuppressive medications associated with decreased responses to routine immunizations? A systematic review. Vaccine. 2012; 30:1413–1424.
Article33. Gisbert JP, Villagrasa JR, Rodríguez-Nogueiras A, Chaparro M. Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am J Gastroenterol. 2012; 107:1460–1466.
Article34. Pratt PK Jr, David N, Weber HC, et al. Antibody response to hepatitis B virus vaccine is impaired in patients with inflammatory bowel disease on infliximab therapy. Inflamm Bowel Dis. 2018; 24:380–386.
Article35. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014; 58:e44–e100. doi: 10.1093/cid/cit684.
Article36. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014; 8:443–468.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of hepatitis B vaccination in patients with ulcerative colitis: a prospective cohort study
- Prevention of Viral Hepatitis and Vaccination
- Viral Hepatitis in Patients with Inflammatory Bowel Disease
- Inflammatory bowel disease is no longer a risk factor of viral hepatitis infection in Asia
- Prevalence of hepatitis B virus and hepatitis C virus infection in patients with inflammatory bowel disease: a systematic review and meta-analysis