Korean J Gastroenterol.
2022 Aug;80(2):51-59. 10.4166/kjg.2022.096.
Viral Hepatitis in Patients with Inflammatory Bowel Disease
- Affiliations
-
- 1Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2532678
- DOI: http://doi.org/10.4166/kjg.2022.096
Abstract
- There has been a rise in the incidence of inflammatory bowel disease (IBD) in developing countries, including South Korea. Consequently, the use of immunosuppressive agents such as immunomodulators or biologics has also increased. Due to immunosuppression, patients on these agents are at increased risk of various opportunistic infections during treatment, which may sometimes lead to serious adverse outcomes. Viral hepatitis, especially hepatitis B, is one of the infectious conditions that can be reactivated during immunosuppressive therapy, and adequate strategies for monitoring and prophylaxis are needed to prevent it. South Korea is one of the countries with intermediate endemicity for hepatitis A and B. Thus, taking adequate precautions against viral hepatitis could prevent new infections or reactivation of these conditions in patients with IBD on immunosuppressive therapy. In this review article, we have summarized the latest evidence on viral hepatitis in patients with IBD that would be of assistance in clinical practice.
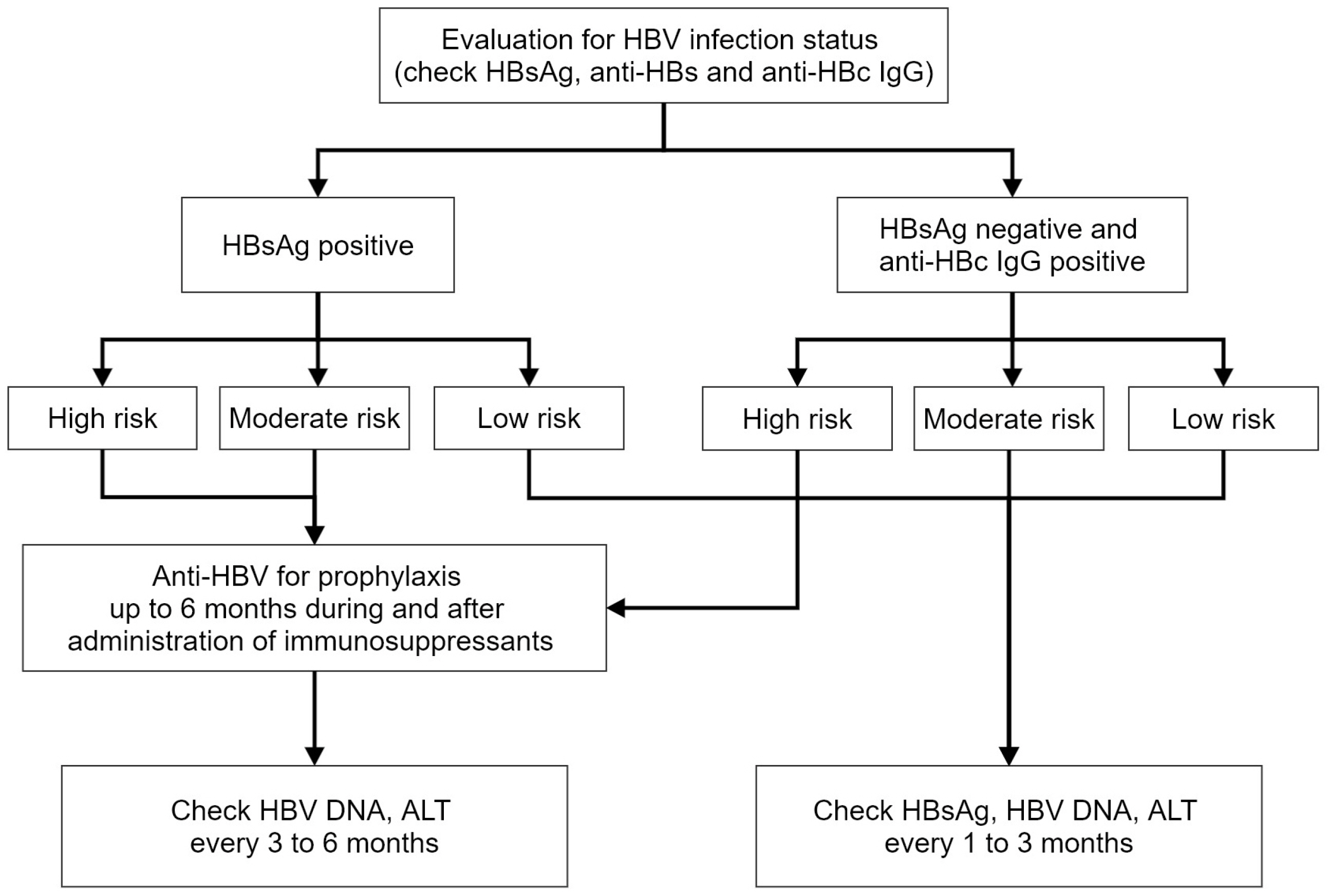
Figure
Reference
-
1. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020; 5:17–30. DOI: 10.1016/S2468-1253(19)30333-4. PMID: 31648971. PMCID: PMC7026709.2. Kwak MS, Cha JM, Lee HH, et al. 2019; Emerging trends of inflammatory bowel disease in South Korea: a nationwide population-based study. J Gastroenterol Hepatol. 34:1018–1026. DOI: 10.1111/jgh.14542. PMID: 30447025.3. Park SH, Kim YJ, Rhee KH, et al. 2019; A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong district of Seoul, Korea in 1986-2015. J Crohns Colitis. 13:1410–1417. DOI: 10.1093/ecco-jcc/jjz081. PMID: 30989166.4. Ng SC, Shi HY, Hamidi N, et al. 2017; Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 390:2769–2778. DOI: 10.1016/S0140-6736(17)32448-0. PMID: 29050646.5. Sood A, Kaur K, Singh A, et al. 2021; Trends of inflammatory bowel disease at a tertiary care center in Northern India. Intest Res. 19:282–290. DOI: 10.5217/ir.2020.00010. PMID: 32806872. PMCID: PMC8322028.6. Singh A, Mahajan R, Kedia S, et al. 2022; Use of thiopurines in inflammatory bowel disease: an update. Intest Res. 20:11–30. DOI: 10.5217/ir.2020.00155. PMID: 33845546. PMCID: PMC8831775.7. Hibi T, Kamae I, Pinton P, et al. 2021; Efficacy of biologic therapies for biologic-naïve Japanese patients with moderately to severely active ulcerative colitis: a network meta-analysis. Intest Res. 19:53–61. DOI: 10.5217/ir.2019.09146. PMID: 32312035. PMCID: PMC7873404.8. Ooi CJ, Hilmi IN, Kim HJ, et al. 2021; Efficacy and safety of vedolizumab in ulcerative colitis in patients from Asian countries in the GEMINI 1 study. Intest Res. 19:71–82. DOI: 10.5217/ir.2019.09159. PMID: 32877600. PMCID: PMC7873399.9. Banerjee R, Chuah SW, Hilmi IN, et al. 2021; Efficacy and safety of vedolizumab in Crohn's disease in patients from Asian countries in the GEMINI 2 study. Intest Res. 19:83–94. DOI: 10.5217/ir.2019.09160. PMID: 33378612. PMCID: PMC7873405.10. Oh SJ, Shin GY, Soh H, et al. 2021; Long-term outcomes of infliximab in a real-world multicenter cohort of patients with acute severe ulcerative colitis. Intest Res. 19:323–331. DOI: 10.5217/ir.2020.00039. PMID: 32806875. PMCID: PMC8322032.11. Hisamatsu T, Kim HJ, Motoya S, et al. 2021; Efficacy and safety of ustekinumab in East Asian patients with moderately to severely active ulcerative colitis: a subpopulation analysis of global phase 3 induction and maintenance studies (UNIFI). Intest Res. 19:386–397. DOI: 10.5217/ir.2020.00080. PMID: 33249802. PMCID: PMC8566834.12. Hisamatsu T, Suzuki Y, Kobayashi M, et al. 2021; Long-term safety and effectiveness of adalimumab in Japanese patients with Crohn's disease: 3-year results from a real-world study. Intest Res. 19:408–418. DOI: 10.5217/ir.2020.00025. PMID: 33207857. PMCID: PMC8566837.13. Ogata H, Hagiwara T, Kawaberi T, Kobayashi M, Hibi T. 2021; Safety and effectiveness of adalimumab in the treatment of ulcerative colitis: results from a large-scale, prospective, multicenter, observational study. Intest Res. 19:419–429. DOI: 10.5217/ir.2020.00033. PMID: 33166442. PMCID: PMC8566831.14. Dave M, Purohit T, Razonable R, Loftus EV Jr. 2014; Opportunistic infections due to inflammatory bowel disease therapy. Inflamm Bowel Dis. 20:196–212. DOI: 10.1097/MIB.0b013e3182a827d2. PMID: 24051931.15. Khanna S. 2021; Management of Clostridioides difficile infection in patients with inflammatory bowel disease. Intest Res. 19:265–274. DOI: 10.5217/ir.2020.00045. PMID: 32806873. PMCID: PMC8322030.16. Kim YS. 2021; Does cytomegalovirus load predict the outcome of acute severe ulcerative colitis? Intest Res. 19:357–359. DOI: 10.5217/ir.2021.00120. PMID: 34731562. PMCID: PMC8566827.17. Kucharzik T, Ellul P, Greuter T, et al. 2021; ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 15:879–913. DOI: 10.1093/ecco-jcc/jjab052. PMID: 33730753.18. Korean Association for the Study of the Liver (KASL). 2022; KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 28:276–331. DOI: 10.3350/cmh.2022.0084. PMID: 35430783. PMCID: PMC9013624.19. Park SK, Choi CH, Chun J, et al. 2020; Prevention and management of viral hepatitis in inflammatory bowel disease: a clinical practice guideline by the Korean Association for the Study of Intestinal Diseases. Intest Res. 18:18–33. DOI: 10.5217/ir.2019.09155. PMID: 32013312. PMCID: PMC7000641.20. Bell BP, Shapiro CN, Alter MJ, et al. 1998; The diverse patterns of hepatitis A epidemiology in the United States-implications for vaccination strategies. J Infect Dis. 178:1579–1584. DOI: 10.1086/314518. PMID: 9815207.21. Moon HW, Cho JH, Hur M, et al. 2010; Laboratory characteristics of recent hepatitis A in Korea: ongoing epidemiological shift. World J Gastroenterol. 16:1115–1118. DOI: 10.3748/wjg.v16.i9.1115. PMID: 20205283. PMCID: PMC2835789.22. Hou JK, Velayos F, Terrault N, Mahadevan U. 2010; Viral hepatitis and inflammatory bowel disease. Inflamm Bowel Dis. 16:925–932. DOI: 10.1002/ibd.21284. PMID: 20480515.23. Hollinger FB, Ticehurst JR, Fields BN, Knipe DM, Howley PM. 1996. Fields virology. 3rd ed. Lippincott-Raven;Philadelphia (PA):24. Korea Centers for Disease Control and Prevention. 2017. Epidemiology and management of vaccine preventable disease. 5th ed. Korea Centers for Disease Control and Prevention;Cheongju:25. Bell BP, Negus S, Fiore AE, et al. 2007; Immunogenicity of an inactivated hepatitis A vaccine in infants and young children. Pediatr Infect Dis J. 26:116–122. DOI: 10.1097/01.inf.0000253253.85640.cc. PMID: 17259872.26. Irving GJ, Holden J, Yang R, Pope D. 2012; Hepatitis A immunisation in persons not previously exposed to hepatitis A. Cochrane Database Syst Rev. 2012:CD009051. DOI: 10.1002/14651858.CD009051.pub2. PMCID: PMC6823267.27. Van Damme P, Banatvala J, Fay O, et al. 2003; Hepatitis A booster vaccination: is there a need? Lancet. 362:1065–1071. DOI: 10.1016/S0140-6736(03)14418-2. PMID: 14522539.28. Park SH, Yang SK, Park SK, et al. 2014; Efficacy of hepatitis A vaccination and factors impacting on seroconversion in patients with inflammatory bowel disease. Inflamm Bowel Dis. 20:69–74. DOI: 10.1097/01.MIB.0000437736.91712.a1. PMID: 24284413.29. Rubin LG, Levin MJ, Ljungman P, et al. 2014; 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 58:309–318. DOI: 10.1093/cid/cit816. PMID: 24421306.30. Global hepatitis report 2017. [Internet]. Available from: https://www.who.int/publications/i/item/9789241565455. Geneva: World Health Organization;2017. Apr. 19. cited 2022 May 21.31. Yim SY, Kim JH. 2019; The epidemiology of hepatitis B virus infection in Korea. Korean J Intern Med. 34:945–953. DOI: 10.3904/kjim.2019.007. PMID: 30919608. PMCID: PMC6718747.32. Korean National Health and Nutrition Examination Survey (KNHANES). 2022. [Internet]. Korea Disease Control and Prevention Agency;Cheongju: Available from: https://knhanes.kdca.go.kr/knhanes/sub04/sub04_04_01.do. cited 2022 May 21.33. Loras C, Saro C, Gonzalez-Huix F, et al. 2009; Prevalence and factors related to hepatitis B and C in inflammatory bowel disease patients in Spain: a nationwide, multicenter study. Am J Gastroenterol. 104:57–63. DOI: 10.1038/ajg.2008.4. PMID: 19098850.34. Park SH, Yang SK, Lim YS, et al. 2012; Clinical courses of chronic hepatitis B virus infection and inflammatory bowel disease in patients with both diseases. Inflamm Bowel Dis. 18:2004–2010. DOI: 10.1002/ibd.22905. PMID: 22337144.35. Huang ML, Xu XT, Shen J, Qiao YQ, Dai ZH, Ran ZH. 2014; Prevalence and factors related to hepatitis B and C infection in inflammatory bowel disease patients in China: a retrospective study. J Crohns Colitis. 8:282–287. DOI: 10.1016/j.crohns.2013.08.017. PMID: 24067604.36. Yeo SJ, Lee HS, Jang BI, et al. 2018; Nonimmunity against hepatitis B virus infection in patients newly diagnosed with inflammatory bowel disease. Intest Res. 16:400–408. DOI: 10.5217/ir.2018.16.3.400. PMID: 30090039. PMCID: PMC6077318.37. Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver JM. 2004; Chronic hepatitis B reactivation following infliximab therapy in Crohn's disease patients: need for primary prophylaxis. Gut. 53:1363–1365. DOI: 10.1136/gut.2004.040675. PMID: 15306601. PMCID: PMC1774200.38. Kim YJ, Bae SC, Sung YK, et al. 2010; Possible reactivation of potential hepatitis B virus occult infection by tumor necrosis factor-alpha blocker in the treatment of rheumatic diseases. J Rheumatol. 37:346–350. DOI: 10.3899/jrheum.090436. PMID: 20008922.39. Alameel T, Al Sulais E. 2021; Risk of HBV reactivation among IBD patients with occult hepatitis B virus infection. Clin Gastroenterol Hepatol. 19:621–622. DOI: 10.1016/j.cgh.2020.04.089. PMID: 33248086.40. Loras C, Gisbert JP, Mínguez M, et al. 2010; Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut. 59:1340–1346. DOI: 10.1136/gut.2010.208413. PMID: 20577000.41. Lee JM, Wei SC, Lee KM, et al. 2022; Clinical course of hepatitis B viral infection in patients undergoing anti-tumor necrosis factor α therapy for inflammatory bowel disease. Gut Liver. 16:396–403. DOI: 10.5009/gnl210081. PMID: 34593670. PMCID: PMC9099383.42. European Association for the Study of the Liver. 2017; EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 67:370–398. DOI: 10.1016/j.jhep.2017.03.021. PMID: 28427875.43. Terrault NA, Lok ASF, McMahon BJ, et al. 2018; Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–1599. DOI: 10.1002/hep.29800. PMID: 29405329. PMCID: PMC5975958.44. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. 2017; ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 112:241–258. DOI: 10.1038/ajg.2016.537. PMID: 28071656.45. World Health Organization. 2019; Hepatitis B vaccines: WHO position paper, July 2017 - Recommendations. Vaccine. 37:223–225. DOI: 10.1016/j.vaccine.2017.07.046. PMID: 28743487.46. Melmed GY, Ippoliti AF, Papadakis KA, et al. 2006; Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 101:1834–1840. DOI: 10.1111/j.1572-0241.2006.00646.x. PMID: 16817843.47. Kochhar GS, Mohan BP, Khan SR, et al. 2021; Hepatitis-B vaccine response in inflammatory bowel disease patients: a systematic review and meta-analysis. Inflamm Bowel Dis. 27:1610–1619. DOI: 10.1093/ibd/izaa353. PMID: 33393585.48. Coates T, Wilson R, Patrick G, André F, Watson V. 2001; Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther. 23:392–403. DOI: 10.1016/S0149-2918(01)80044-8. PMID: 11318074.49. Jiang HY, Wang SY, Deng M, et al. 2017; Immune response to hepatitis B vaccination among people with inflammatory bowel diseases: a systematic review and meta-analysis. Vaccine. 35:2633–2641. DOI: 10.1016/j.vaccine.2017.03.080. PMID: 28404358.50. Gisbert JP, Menchén L, García-Sánchez V, Marín I, Villagrasa JR, Chaparro M. 2012; Comparison of the effectiveness of two protocols for vaccination (standard and double dosage) against hepatitis B virus in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 35:1379–1385. DOI: 10.1111/j.1365-2036.2012.05110.x. PMID: 22530631.51. Korean Association for the Study of the Liver (KASL). 2019; KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 25:93–159. DOI: 10.3350/cmh.2019.1002. PMID: 31185710. PMCID: PMC6589848.52. Cossio-Gil Y, Martínez-Gómez X, Campins-Martí M, et al. 2015; Immunogenicity of hepatitis B vaccine in patients with inflammatory bowel disease and the benefits of revaccination. J Gastroenterol Hepatol. 30:92–98. DOI: 10.1111/jgh.12712. PMID: 25160690.53. Pratt PK Jr, Nunes D, Long MT, Farraye FA. 2019; Improved antibody response to three additional hepatitis B vaccine doses following primary vaccination failure in patients with inflammatory bowel disease. Dig Dis Sci. 64:2031–2038. DOI: 10.1007/s10620-019-05595-6. PMID: 30945037. PMCID: PMC6764090.54. Gisbert JP, Villagrasa JR, Rodríguez-Nogueiras A, Chaparro M. 2013; Kinetics of anti-hepatitis B surface antigen titers after hepatitis B vaccination in patients with inflammatory bowel disease. Inflamm Bowel Dis. 19:554–558. DOI: 10.1097/MIB.0b013e31827febe9. PMID: 23380936.55. Perrillo RP, Gish R, Falck-Ytter YT. 2015; American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 148:221–244.e3. DOI: 10.1053/j.gastro.2014.10.038. PMID: 25447852.56. Loomba R, Liang TJ. 2017; Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 152:1297–1309. DOI: 10.1053/j.gastro.2017.02.009. PMID: 28219691. PMCID: PMC5501983.57. Lan JL, Chen YM, Hsieh TY, et al. 2011; Kinetics of viral loads and risk of hepatitis B virus reactivation in hepatitis B core antibody-positive rheumatoid arthritis patients undergoing anti-tumour necrosis factor alpha therapy. Ann Rheum Dis. 70:1719–1725. DOI: 10.1136/ard.2010.148783. PMID: 21719446.58. Aygen B, Demir AM, Gümüş M, et al. 2018; Immunosuppressive therapy and the risk of hepatitis B reactivation: consensus report. Turk J Gastroenterol. 29:259–269. DOI: 10.5152/tjg.2018.18263. PMID: 29755010. PMCID: PMC6284666.59. Ahn SM, Choi J, Ye BD, et al. 2022; Risk of Hepatitis B virus (HBV) reactivation in patients with immune-mediated inflammatory diseases receiving biologics: focus on the timing of biologics after anti-HBV treatment. Gut Liver. 16:567–574. DOI: 10.5009/gnl210204. PMID: 34840146. PMCID: PMC9289826.60. Ting SW, Chen YC, Huang YH. 2018; Risk of hepatitis B reactivation in patients with psoriasis on ustekinumab. Clin Drug Investig. 38:873–880. DOI: 10.1007/s40261-018-0671-z. PMID: 29968197.61. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT. American Gastroenterological Association Institute. 2015; American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 148:215–e17. DOI: 10.1053/j.gastro.2014.10.039. PMID: 25447850.62. Madonia S, Orlando A, Scimeca D, Olivo M, Rossi F, Cottone M. 2007; Occult hepatitis B and infliximab-induced HBV reactivation. Inflamm Bowel Dis. 13:508–509. DOI: 10.1002/ibd.20035. PMID: 17206687.63. Axiaris G, Zampeli E, Michopoulos S, Bamias G. 2021; Management of hepatitis B virus infection in patients with inflammatory bowel disease under immunosuppressive treatment. World J Gastroenterol. 27:3762–3779. DOI: 10.3748/wjg.v27.i25.3762. PMID: 34321842. PMCID: PMC8291024.64. Millman AJ, Nelson NP, Vellozzi C. 2017; Hepatitis C: review of the epidemiology, clinical care, and continued challenges in the direct acting antiviral era. Curr Epidemiol Rep. 4:174–185. DOI: 10.1007/s40471-017-0108-x. PMID: 28785531. PMCID: PMC5544136.65. Chevaux JB, Nani A, Oussalah A, et al. 2010; Prevalence of hepatitis B and C and risk factors for nonvaccination in inflammatory bowel disease patients in Northeast France. Inflamm Bowel Dis. 16:916–924. DOI: 10.1002/ibd.21147. PMID: 19885908.66. Harsh P, Gupta V, Kedia S, et al. 2017; Prevalence of hepatitis B, hepatitis C and human immunodeficiency viral infections in patients with inflammatory bowel disease in north India. Intest Res. 15:97–102. DOI: 10.5217/ir.2017.15.1.97. PMID: 28239319. PMCID: PMC5323314.67. Korean Association for the Study of the Liver. 2016; KASL clinical practice guidelines: management of hepatitis C. Clin Mol Hepatol. 22:76–139. DOI: 10.3350/cmh.2016.22.1.76. PMID: 27044763. PMCID: PMC4825161.68. Ghany MG, Morgan TR. AASLD-IDSA Hepatitis C Guidance Panel. 2020; Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 71:686–721. DOI: 10.1002/hep.31060. PMID: 31816111.69. European Association for the Study of the Liver. 2020; EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 73:1170–1218. DOI: 10.1016/j.jhep.2020.08.018. PMID: 32956768.70. Cardona AM, Horta D, Florez-Diez P, et al. 2022; Evaluation of the safety and effectiveness of direct-acting antiviral drugs in the treatment of hepatitis C in patients with inflammatory bowel disease: national multicenter study (ENEIDA registry). MIC project. Journal of Crohns & Colitis. 16(Suppl 1):i321–i322. DOI: 10.1093/ecco-jcc/jjab232.416.71. Korean Association for the Study of the Liver (KASL). 2018; 2017 KASL clinical practice guidelines management of hepatitis C: treatment of chronic hepatitis C. Clin Mol Hepatol. 24:169–229. DOI: 10.3350/cmh.2018.1004. PMID: 30092624. PMCID: PMC6166104.72. Morisco F, Castiglione F, Rispo A, et al. 2013; Effect of immunosuppressive therapy on patients with inflammatory bowel diseases and hepatitis B or C virus infection. J Viral Hepat. 20:200–208. DOI: 10.1111/j.1365-2893.2012.01643.x. PMID: 23383659.73. Papa A, Felice C, Marzo M, et al. 2013; Prevalence and natural history of hepatitis B and C infections in a large population of IBD patients treated with anti-tumor necrosis factor-α agents. J Crohns Colitis. 7:113–119. DOI: 10.1016/j.crohns.2012.03.001. PMID: 22464811.74. Loras C, Gisbert JP, Saro MC, et al. 2014; Impact of surveillance of hepatitis b and hepatitis c in patients with inflammatory bowel disease under anti-TNF therapies: multicenter prospective observational study (REPENTINA 3). J Crohns Colitis. 8:1529–1538. DOI: 10.1016/j.crohns.2014.06.009. PMID: 25052345.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inflammatory bowel disease is no longer a risk factor of viral hepatitis infection in Asia
- Evidence-based consensus on opportunistic infections in inflammatory bowel disease (republication)
- Prevention of Viral Hepatitis and Vaccination
- Prevalence of hepatitis B virus and hepatitis C virus infection in patients with inflammatory bowel disease: a systematic review and meta-analysis
- Prevalence of hepatitis B, hepatitis C and human immunodeficiency viral infections in patients with inflammatory bowel disease in north India