Obstet Gynecol Sci.
2017 Mar;60(2):154-162. 10.5468/ogs.2017.60.2.154.
Maternal serum placental growth factor and pregnancy-associated plasma protein A measured in the first trimester as parameters of subsequent pre-eclampsia and small-for-gestational-age infants: A prospective observational study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, National Health Insurance Service Ilsan Hospital, Goyang, Korea. raksumi10@gmail.com
- KMID: 2372799
- DOI: http://doi.org/10.5468/ogs.2017.60.2.154
Abstract
OBJECTIVE
To examine the first-trimester maternal serum placental growth factor (PlGF) and pregnancy-associated plasma protein A (PAPP-A) levels in pregnancies associated with pre-eclampsia (PE) or small-for-gestational-age (SGA) infants, and determine the predictive accuracy of PlGF and of PAPP-A for either PE or SGA infants.
METHODS
This prospective, observational study included 175 pregnant women, and of these women, due to participant withdrawal or loss to follow-up, delivery data were collected from the medical records of 155 women, including 4 who had twin pregnancies. The women's maternal history was recorded, and the PlGF and PAPP-A levels at 11 to 13 gestational weeks were measured. During the second trimester, the maternal uterine artery's systolic/diastolic ratio was measured. Multiples of the median (MoM) of PlGF and PAPP-A were determined, and the associations of these values with the risk factors of SGA and PE were evaluated. Logistic regression analysis was used to determine whether PlGF and PAPP-A are useful markers for predicting SGA infants.
RESULTS
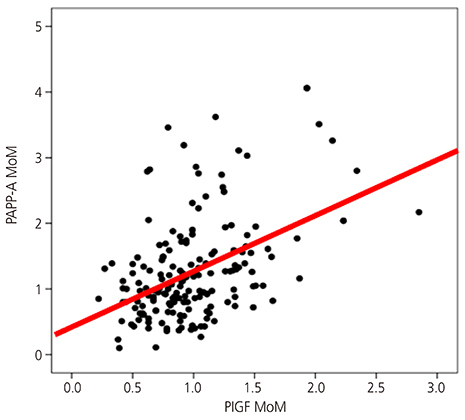
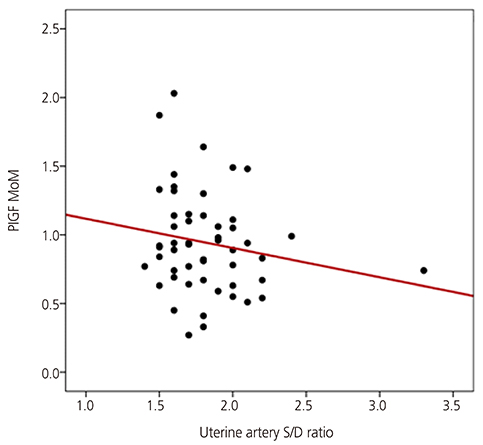
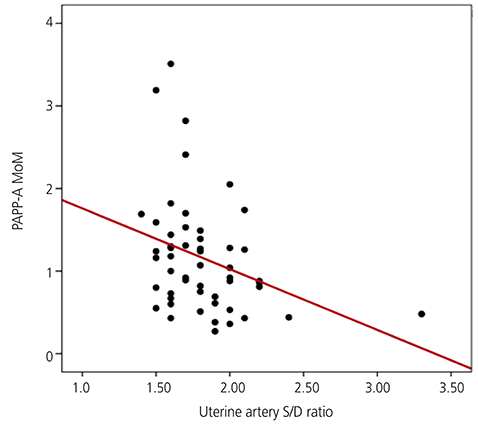
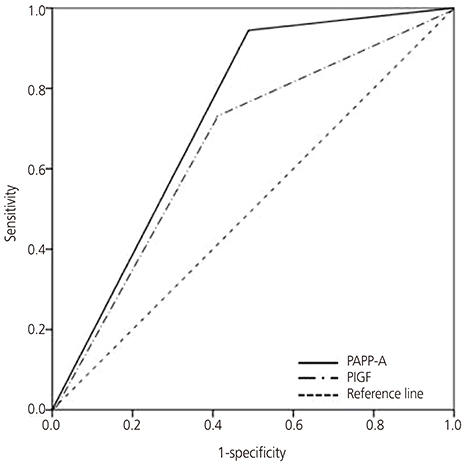
The PAPP-A MoM level was significantly lower in women with advanced maternal age, multipara women, and women with gestational diabetes than in their counterparts. The PlGF and PAPP-A MoM levels were higher in women with a twin pregnancy than in those with a singleton pregnancy. There was a significant relationship between the maternal serum PAPP-A MoM level in the first trimester and the uterine artery systolic/diastolic ratio in the second trimester. Results of logistic regression analysis showed that low PlGF and PAPP-A MoM levels were predictors of SGA infants (odds ratio, 0.143; 95% confidence interval, 0.025 to 0.806; odds ratio, 0.191; 95% confidence interval, 0.051 to 0.718, respectively).
CONCLUSION
PlGF and PAPP-A are potentially useful as first-trimester markers for SGA infants and some hypertensive disorders of pregnancy.
Keyword
MeSH Terms
-
Diabetes, Gestational
Female
Follow-Up Studies
Humans
Infant*
Logistic Models
Maternal Age
Medical Records
Observational Study*
Odds Ratio
Plasma*
Pre-Eclampsia*
Pregnancy
Pregnancy Trimester, First*
Pregnancy Trimester, Second
Pregnancy, Twin
Pregnancy-Associated Plasma Protein-A
Pregnant Women
Prospective Studies*
Risk Factors
Staphylococcal Protein A*
Uterine Artery
Pregnancy-Associated Plasma Protein-A
Staphylococcal Protein A
Figure
Cited by 1 articles
-
The evaluating of pregnancy-associated plasma protein-A with the likelihood of small for gestational age
Maryam Sadat Hoseini, Samaneh Sheibani, Mehrdad Sheikhvatan
Obstet Gynecol Sci. 2020;63(3):225-230. doi: 10.5468/ogs.2020.63.3.225.
Reference
-
1. Sibai BM, Caritis S, Hauth J. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. What we have learned about preeclampsia. Semin Perinatol. 2003; 27:239–246.2. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011; 25:391–403.3. Madazli R, Budak E, Calay Z, Aksu MF. Correlation between placental bed biopsy findings, vascular cell adhesion molecule and fibronectin levels in pre-eclampsia. BJOG. 2000; 107:514–518.4. Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001; 357:209–215.5. Jackson MR, Walsh AJ, Morrow RJ, Mullen JB, Lye SJ, Ritchie JW. Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery Doppler waveforms. Am J Obstet Gynecol. 1995; 172:518–525.6. Cowans NJ, Stamatopoulou A, Matwejew E, von Kaisenberg CS, Spencer K. First-trimester placental growth factor as a marker for hypertensive disorders and SGA. Prenat Diagn. 2010; 30:565–570.7. Poon LC, Zaragoza E, Akolekar R, Anagnostopoulos E, Nicolaides KH. Maternal serum placental growth factor (PlGF) in small for gestational age pregnancy at 11(+0) to 13(+6) weeks of gestation. Prenat Diagn. 2008; 28:1110–1115.8. Kasdaglis T, Aberdeen G, Turan O, Kopelman J, Atlas R, Jenkins C, et al. Placental growth factor in the first trimester: relationship with maternal factors and placental Doppler studies. Ultrasound Obstet Gynecol. 2010; 35:280–285.9. Torry DS, Ahn H, Barnes EL, Torry RJ. Placenta growth factor: potential role in pregnancy. Am J Reprod Immunol. 1999; 41:79–85.10. Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991; 88:9267–9271.11. Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997; 18:657–665.12. Vuorela P, Hatva E, Lymboussaki A, Kaipainen A, Joukov V, Persico MG, et al. Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol Reprod. 1997; 56:489–494.13. Brambati B, Macintosh MC, Teisner B, Maguiness S, Shrimanker K, Lanzani A, et al. Low maternal serum levels of pregnancy associated plasma protein A (PAPP-A) in the first trimester in association with abnormal fetal karyotype. Br J Obstet Gynaecol. 1993; 100:324–326.14. Yaron Y, Heifetz S, Ochshorn Y, Lehavi O, Orr-Urtreger A. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenat Diagn. 2002; 22:778–782.15. Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002; 87:1762–1767.16. Todros T, Sciarrone A, Piccoli E, Guiot C, Kaufmann P, Kingdom J. Umbilical Doppler waveforms and placental villous angiogenesis in pregnancies complicated by fetal growth restriction. Obstet Gynecol. 1999; 93:499–503.17. Lounghna P, Chitty L, Evans T, Chudleigh T. Fetal sized and dating: charts recommended for clinical obstetric practice. Ultrasound. 2009; 17:160–166.18. Lee JJ. Birth weight for gestational age patterns by sex, plurality, and parity in Korean population. Korean J Pediatr. 2007; 50:732–739.19. Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008; 21:279–287.20. Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007; 50:137–142.21. Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008; 21:9–23.22. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982; 142:159–167.23. Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993; 341:938–941.24. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005; 365:785–799.25. Curtin WM, Weinstein L. A review of HELLP syndrome. J Perinatol. 1999; 19:138–143.26. Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001; 357:53–56.27. Mihu D, Costin N, Mihu CM, Seicean A, Ciortea R. HELLP syndrome: a multisystemic disorder. J Gastrointestin Liver Dis. 2007; 16:419–424.28. Sibai BM, Koch MA, Freire S, Pinto e Silva JL, Rudge MV, Martins-Costa S, et al. Serum inhibin A and angiogenic factor levels in pregnancies with previous preeclampsia and/or chronic hypertension: are they useful markers for prediction of subsequent preeclampsia? Am J Obstet Gynecol. 2008; 199:268.29. Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007; 196:239.30. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004; 350:672–683.31. Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004; 89:770–775.32. Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001; 184:1267–1272.33. Smith GC, Crossley JA, Aitken DA, Jenkins N, Lyall F, Cameron AD, et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007; 109:1316–1324.34. Vatten LJ, Asvold BO, Eskild A. Angiogenic factors in maternal circulation and preeclampsia with or without fetal growth restriction. Acta Obstet Gynecol Scand. 2012; 91:1388–1394.35. Ong CY, Liao AW, Spencer K, Munim S, Nicolaides KH. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG. 2000; 107:1265–1270.36. Sanchez O, Llurba E, Marsal G, Dominguez C, Aulesa C, Sanchez-Duran MA, et al. First trimester serum angiogenic/anti-angiogenic status in twin pregnancies: relationship with assisted reproduction technology. Hum Reprod. 2012; 27:358–365.37. Svirsky R, Levinsohn-Tavor O, Feldman N, Klog E, Cuckle H, Maymon R. First- and second-trimester maternal serum markers of pre-eclampsia in twin pregnancy. Ultrasound Obstet Gynecol. 2016; 47:560–564.38. Chasen ST, Martinucci S, Perni SC, Kalish RB. First-trimester biochemistry and outcomes in twin pregnancy. J Reprod Med. 2009; 54:312–314.39. Rochelson BL, Schulman H, Fleischer A, Farmakides G, Bracero L, Ducey J, et al. The clinical significance of Doppler umbilical artery velocimetry in the small for gestational age fetus. Am J Obstet Gynecol. 1987; 156:1223–1226.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Midtrimester maternal plasma concentrations of angiopoietin 1, angiopoietin 2, and placental growth factor in pregnant women who subsequently develop preeclampsia
- Maternal Plasma Homocysteine Levels and Pregnancy Outcomes
- A study of the factors associated with the pattern of gestational weight gain
- The association of serum placental growth factor with pregnancies complicated by preeclampsia and small for gestational age
- Urinary nephrin: A new predictive marker for pregnancies with preeclampsia and small-for-gestational age infants