Obstet Gynecol Sci.
2013 Jan;56(1):22-28. 10.5468/OGS.2013.56.1.22.
Urinary nephrin: A new predictive marker for pregnancies with preeclampsia and small-for-gestational age infants
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Ewha Womans University School of Medicine, Seoul, Korea. kkyj@ewha.ac.kr
- 2Department of Obstetrics and Gynecology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 3Medical Research Institute, Ewha Womans University, Seoul, Korea.
- 4Department of Preventive Medicine, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 1857182
- DOI: http://doi.org/10.5468/OGS.2013.56.1.22
Abstract
OBJECTIVE
The objective of this study was to determine the differences in urinary nephrin among controls, gravidas with preeclampsia (PE), and small-for-gestational age (SGA) infants. We also determined whether or not maternal urinary concentrations of nephrin are associated with the subsequent development of PE and SGA infants.
METHODS
We analyzed maternal urinary levels of nephrin in women who were normal controls (n=50), women who were delivered SGA infants (n=40), and gravidas with PE (n=33) in the first, second and third trimesters. Urinary nephrin concentrations were measured with nephrin enzyme-linked immunosorbent assay kits.
RESULTS
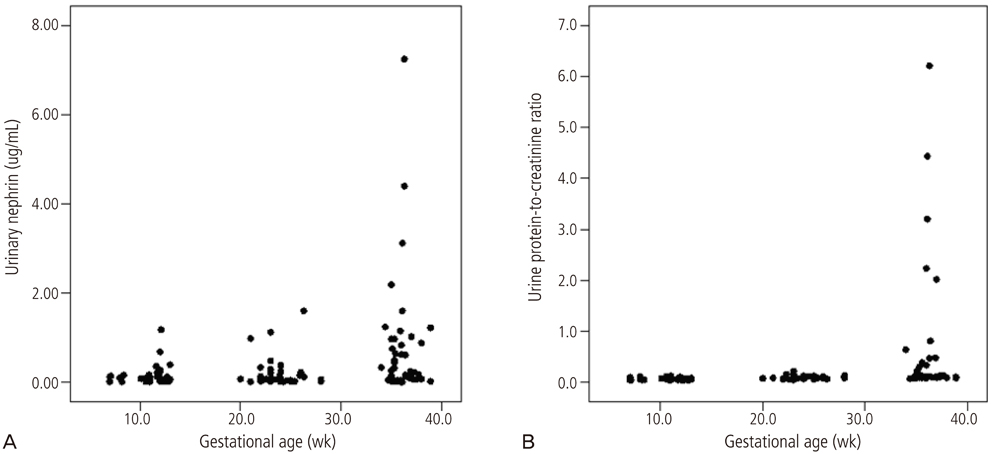
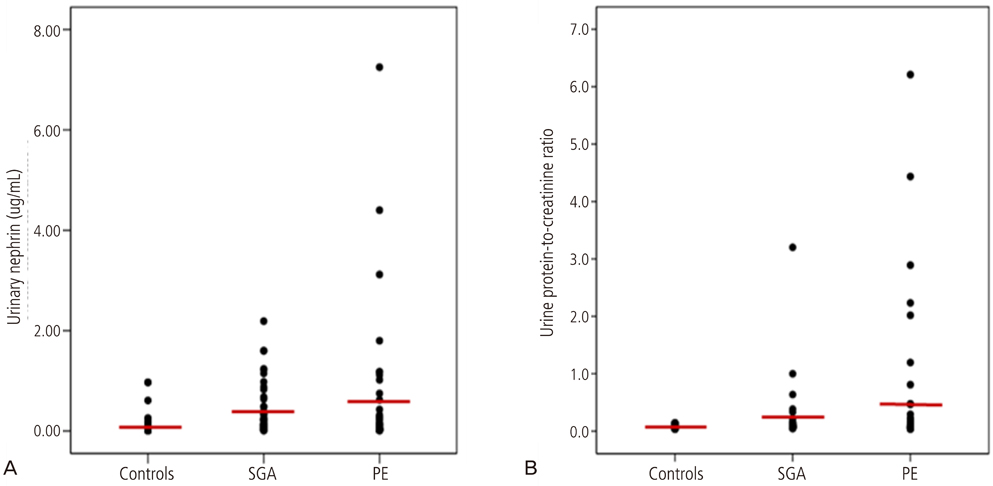
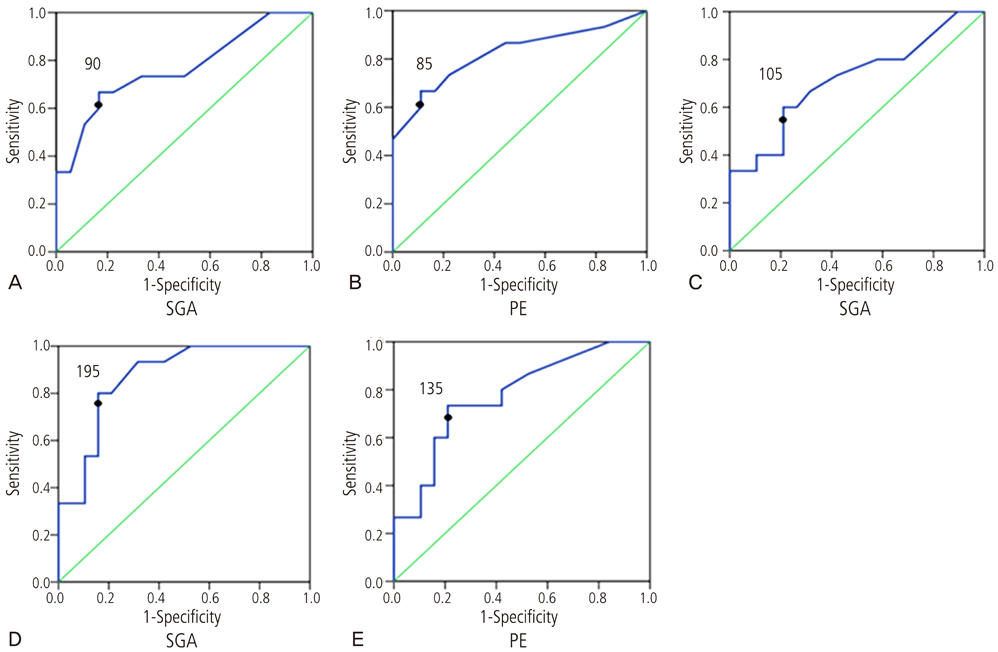
The levels of urinary nephrin were higher in gravida developing preeclampsia or SGA than in controls after adjusting serum creatinine (P<0.05 for both). Maternal urine concentrations of nephrin were higher in pregnancies complicated by SGA and PE in the third trimester (P<0.05), and also higher in pregnancies complicated by SGA in the first trimester (P<0.05). The sensitivity and specificity of nephrin in predicting SGA from normal pregnancies were 67% and 89% in the first trimester, 60% and 79% in the second trimester, and 80% and 84% in the third trimester, respectively. The sensitivity and specificity of nephrin in predicting PE from normal pregnancies were 67% and 83% in the first trimester and 73% and 79% in the third trimester, respectively.
CONCLUSION
We suggest that urinary nephrin can be used as an early marker in pregnancies at risk for developing PE and SGA infants.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee JJ. Birth weight for gestational age patterns by sex, plurality, and parity in Korean populations. Korean J Perinatol. 2007. 18:1–11.2. Garovic VD, Wagner SJ, Turner ST, Rosenthal DW, Watson WJ, Brost BC, et al. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol. 2007. 196:320.e1–320.e7.3. Zhao S, Gu X, Groome LJ, Wang Y. Decreased nephrin and GLEPP-1, but increased VEGF, Flt-1, and nitrotyrosine, expressions in kidney tissue sections from women with preeclampsia. Reprod Sci. 2009. 16:970–979.4. Karumanchi SA, Lindheimer MD. Preeclampsia and the kidney: footprints in the urine. Am J Obstet Gynecol. 2007. 196:287–288.5. Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998. 1:575–582.6. Lenkkeri U, Mannikko M, McCready P, Lamerdin J, Gribouval O, Niaudet PM, et al. Structure of the gene for congenital nephrotic syndrome of the finnish type (NPHS1) and characterization of mutations. Am J Hum Genet. 1999. 64:51–61.7. Patrakka J, Tryggvason K. Nephrin: a unique structural and signaling protein of the kidney filter. Trends Mol Med. 2007. 13:396–403.8. Son GH, Kim JH, Hwang JH, Kim YH, Park YW, Kwon JY. Urinary excretion of nephrin in patients with severe preeclampsia. Urinary nephrin in preeclampsia. Hypertens Pregnancy. 2011. 30:408–413.9. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000. 183:S1–S22.10. Zhao S, Gu Y, Coates G, Groome LJ, Saleem MA, Mathieson PW, et al. Altered nephrin and podoplanin distribution is associated with disturbed polarity protein PARD-3 and PARD-6 expressions in podocytes from preeclampsia. Reprod Sci. 2011. 18:772–780.11. Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, et al. Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol. 2009. 20:1041–1052.12. Garovic VD, Wagner SJ, Petrovic LM, Gray CE, Hall P, Sugimoto H, et al. Glomerular expression of nephrin and synaptopodin, but not podocin, is decreased in kidney sections from women with preeclampsia. Nephrol Dial Transplant. 2007. 22:1136–1143.13. Collino F, Bussolati B, Gerbaudo E, Marozio L, Pelissetto S, Benedetto C, et al. Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol. 2008. 294:F1185–F1194.14. Beall MH, Amidi F, Gayle DA, Wang S, Beloosesky R, Ross MG. Placental and fetal membrane Nephrin and Neph1 gene expression: response to inflammation. J Soc Gynecol Investig. 2005. 12:298–302.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Level of Serum and Urinary Nephrin in Normal Pregnancy and Pregnancy with Subsequent Preeclampsia
- Difference in serum nephrin expression between normal and preeclamptic pregnancies: A preliminary study
- Efficacy of midtrimester amniotic fluid 8-isoprostane measurement in the prediction of severe preeclampsia
- The association of serum placental growth factor with pregnancies complicated by preeclampsia and small for gestational age
- Relationships between TNF-alpha and fetal growth restriction in preeclamptic women and normotensive pregnancies