Obstet Gynecol Sci.
2017 Mar;60(2):145-153. 10.5468/ogs.2017.60.2.145.
Association of citalopram with congenital anomalies: A meta-analysis
- Affiliations
-
- 1Education and Human Resources Department, Samsung Medical Center, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea. novak082@naver.com
- 3Department of Pediatrics, Korea University College of Medicine, Seoul, Korea.
- 4Clinical Research Center, Asan Medical Center, Seoul, Korea.
- KMID: 2372798
- DOI: http://doi.org/10.5468/ogs.2017.60.2.145
Abstract
OBJECTIVE
The antenatal use of citalopram, a widely prescribed selective serotonin reuptake inhibitor, has been suspected to be associated with congenital, particularly cardiac, anomalies. This study aimed to prove the association between citalopram use and congenital anomalies.
METHODS
We searched the English literature from July 1998 to July 2015, by using the search terms "˜ citalopram', "˜ pregnancy', "˜ birth defects', "˜ congenital anomalies', and "˜ malformations' in PubMed, Embase, Web of Science, and the Cochrane Library.
RESULTS
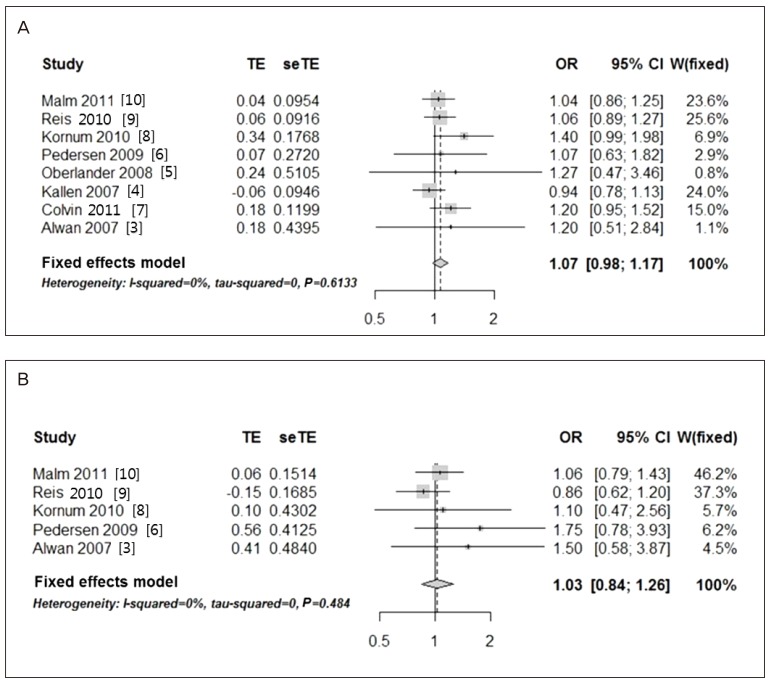
Eight eligible articles were analyzed including a total of 1,507,896 participants. The odds ratio (OR) of major malformations associated with citalopram use during pregnancy was 1.07 (95% confidence interval [CI], 0.98 to 1.17). Concerning cardiac malformations, the OR for all included studies was 1.31 (95% CI, 0.88 to 1.93). The analysis of cardiac malformations was repeated to reduce heterogeneity after excluding one outlier study (OR, 1.03; 95% CI, 0.84 to 1.26).
CONCLUSION
From our data, it can be concluded that citalopram use is not associated with major birth defects. However, physicians should carefully weigh the benefits against the potential risks of citalopram use, and counsel patients accordingly.
MeSH Terms
Figure
Reference
-
1. Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006; 295:499–507. PMID: 16449615.
Article2. Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008; 198:194. PMID: 17905176.
Article3. Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007; 356:2684–2692. PMID: 17596602.
Article4. Kallen BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007; 79:301–308. PMID: 17216624.5. Oberlander TF, Warburton W, Misri S, Riggs W, Aghajanian J, Hertzman C. Major congenital malformations following prenatal exposure to serotonin reuptake inhibitors and benzodiazepines using population-based health data. Birth Defects Res B Dev Reprod Toxicol. 2008; 83:68–76. PMID: 18293409.
Article6. Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009; 339:b3569. PMID: 19776103.
Article7. Colvin L, Slack-Smith L, Stanley FJ, Bower C. Dispensing patterns and pregnancy outcomes for women dispensed selective serotonin reuptake inhibitors in pregnancy. Birth Defects Res A Clin Mol Teratol. 2011; 91:142–152. PMID: 21381184.
Article8. Kornum JB, Nielsen RB, Pedersen L, Mortensen PB, Norgaard M. Use of selective serotonin-reuptake inhibitors during early pregnancy and risk of congenital malformations: updated analysis. Clin Epidemiol. 2010; 2:29–36. PMID: 20865100.
Article9. Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med. 2010; 40:1723–1733. PMID: 20047705.
Article10. Malm H, Artama M, Gissler M, Ritvanen A. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet Gynecol. 2011; 118:111–120. PMID: 21646927.
Article11. Nikfar S, Rahimi R, Hendoiee N, Abdollahi M. Increasing the risk of spontaneous abortion and major malformations in newborns following use of serotonin reuptake inhibitors during pregnancy: a systematic review and updated meta-analysis. Daru. 2012; 20:75. PMID: 23351929.
Article12. Myles N, Newall H, Ward H, Large M. Systematic meta-analysis of individual selective serotonin reuptake inhibitor medications and congenital malformations. Aust N Z J Psychiatry. 2013; 47:1002–1012. PMID: 23761574.
Article13. Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009; 373:746–758. PMID: 19185342.
Article14. Hellerstein DJ, Batchelder S, Miozzo R, Kreditor D, Hyler S, Gangure D, et al. Citalopram in the treatment of dysthymic disorder. Int Clin Psychopharmacol. 2004; 19:143–148. PMID: 15107656.
Article15. Varia I, Rauscher F. Treatment of generalized anxiety disorder with citalopram. Int Clin Psychopharmacol. 2002; 17:103–107. PMID: 11981350.
Article16. Stein DJ, Montgomery SA, Kasper S, Tanghoj P. Predictors of response to pharmacotherapy with citalopram in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2001; 16:357–361. PMID: 11712625.
Article17. Heikkinen T, Ekblad U, Kero P, Ekblad S, Laine K. Citalopram in pregnancy and lactation. Clin Pharmacol Ther. 2002; 72:184–191. PMID: 12189365.
Article18. Shuey DL, Sadler TW, Tamir H, Lauder JM. Serotonin and morphogenesis: transient expression of serotonin uptake and binding protein during craniofacial morphogenesis in the mouse. Anat Embryol (Berl). 1993; 187:75–85. PMID: 8430902.19. Yavarone MS, Shuey DL, Tamir H, Sadler TW, Lauder JM. Serotonin and cardiac morphogenesis in the mouse embryo. Teratology. 1993; 47:573–584. PMID: 8367830.
Article20. Sari Y, Zhou FC. Serotonin and its transporter on proliferation of fetal heart cells. Int J Dev Neurosci. 2003; 21:417–424. PMID: 14659992.
Article21. Committee on Drugs. American Academy of Pediatrics. Use of psychoactive medication during pregnancy and possible effects on the fetus and newborn. Pediatrics. 2000; 105:880–887. PMID: 10742343.22. Hiemke C, Hartter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000; 85:11–28. PMID: 10674711.
Article23. Milne RJ, Goa KL. Citalopram: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depressive illness. Drugs. 1991; 41:450–477. PMID: 1711447.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Congenital hand differences: a comprehensive literature review
- Environmental and Genetic Risk Factors of Congenital Anomalies: an Umbrella Review of Systematic Reviews and Meta-Analyses
- Does antiretroviral therapy cause congenital malformations? A systematic review and meta-analysis
- Clinical Features and Musculoskeletal Anomalies in VATER Association
- Two Cases of Citalopram Induced Awake Bruxism