J Korean Med Sci.
2021 Jul;36(28):e183. 10.3346/jkms.2021.36.e183.
Environmental and Genetic Risk Factors of Congenital Anomalies: an Umbrella Review of Systematic Reviews and Meta-Analyses
- Affiliations
-
- 1Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea
- 2Environmental Health Center, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Statistics, University of Pittsburgh, Pittsburgh, PA, USA
- 4Institute of Environmental Medicine, Seoul National University Medical Research Center, Seoul, Korea
- 5Department of Surgery, Wonkwang University Sanbon Hospital, Gunpo, Korea
- 6Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2518523
- DOI: http://doi.org/10.3346/jkms.2021.36.e183
Abstract
- Background
The prevalence of congenital anomalies in newborns in South Korea was 272.9 per 100,000 in 2005, and 314.7 per 100,000 in 2006. In other studies, the prevalence of congenital anomalies in South Korea was equivalent to 286.9 per 10,000 livebirths in 2006, while it was estimated 446.3 per 10,000 births during the period from 2008 to 2014. Several systematic reviews and meta-analyses analyzing the factors contributing to congenital anomalies have been reported, but comprehensive umbrella reviews are lacking.
Methods
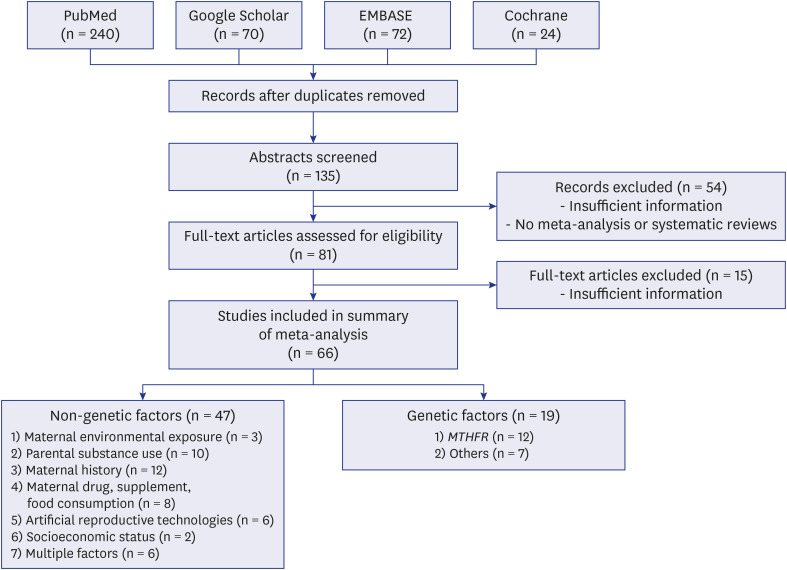
We searched PubMed, Google Scholar, Cochrane, and EMBASE databases up to July 1, 2019, for systematic reviews and meta-analyses that investigated the effects of environmental and genetic factors on any type of congenital anomalies. We categorized 8 subgroups of congenital anomalies classified according to the 10th revision of the International Statistical Classification of Diseases (ICD-10). Two researchers independently searched the literature, retrieved the data, and evaluated the quality of each study.
Results
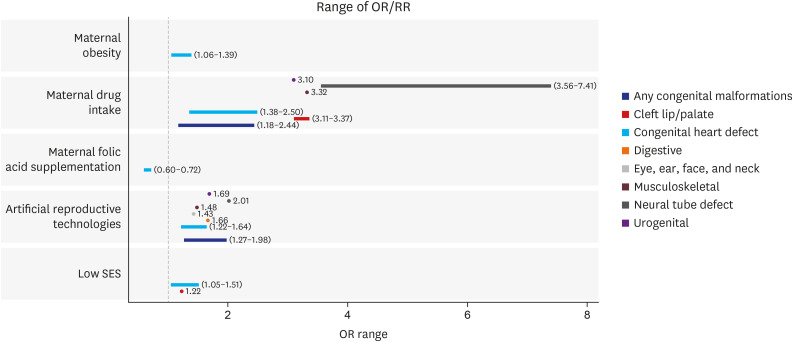
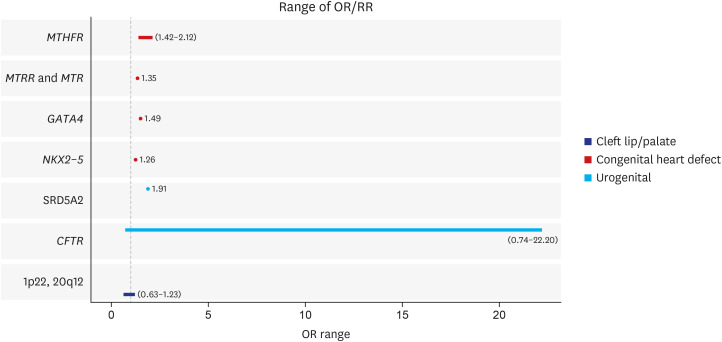
We reviewed 66 systematic reviews and meta-analyses that investigated the association between non-genetic or genetic risk factors and congenital anomalies. Overall, 269 associations and 128 associations were considered for environmental and genetic risk factors, respectively. Congenital anomalies based on congenital heart diseases, cleft lip and palate, and others were associated with environmental risk factors based on maternal exposure to environmental exposures (air pollution, toxic chemicals), parental smoking, maternal history (infectious diseases during pregnancy, pregestational and gestational diabetes mellitus, and gestational diabetes mellitus), maternal obesity, maternal drug intake, pregnancy through artificial reproductive technologies, and socioeconomic factors. The association of maternal alcohol or coffee consumption with congenital anomalies was not significant, and maternal folic acid supplementation had a preventive effect on congenital heart defects. Genes or genetic loci associated with congenital anomalies included MTHFR, MTRR and MTR, GATA4, NKX2-5, SRD5A2, CFTR, and 1p22 and 20q12 anomalies.
Conclusion
This study provides a wide perspective on the distribution of environmental and genetic risk factors of congenital anomalies, thus suggesting future studies and providing health policy implications.
Keyword
Figure
Reference
-
1. Lim JW. The changing trends in live birth statistics in Korea, 1970 to 2010. Korean J Pediatr. 2011; 54(11):429–435. PMID: 22253639.
Article2. Kim MA, Yee NH, Choi JS, Choi JY, Seo K. Prevalence of birth defects in Korean livebirths, 2005–2006. J Korean Med Sci. 2012; 27(10):1233–1240. PMID: 23091323.
Article3. Ko JK, Lamichhane DK, Kim HC, Leem JH. Trends in the prevalences of selected birth defects in Korea (2008–2014). Int J Environ Res Public Health. 2018; 15(5):923–936.
Article4. Boyle B, Addor MC, Arriola L, Barisic I, Bianchi F, Csáky-Szunyogh M, et al. Estimating Global Burden of Disease due to congenital anomaly: an analysis of European data. Arch Dis Child Fetal Neonatal Ed. 2018; 103(1):F22–8. PMID: 28667189.
Article5. Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol. 2010; 686(1):349–364. PMID: 20824455.
Article6. de Hundt M, Vlemmix F, Bais JM, Hutton EK, de Groot CJ, Mol BW, et al. Risk factors for developmental dysplasia of the hip: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2012; 165(1):8–17. PMID: 22824571.
Article7. Ortiz-Neira CL, Paolucci EO, Donnon T. A meta-analysis of common risk factors associated with the diagnosis of developmental dysplasia of the hip in newborns. Eur J Radiol. 2012; 81(3):e344–51. PMID: 22119556.
Article8. Zwink N, Jenetzky E, Brenner H. Parental risk factors and anorectal malformations: systematic review and meta-analysis. Orphanet J Rare Dis. 2011; 6(1):25. PMID: 21586115.
Article9. Oldereid NB, Wennerholm UB, Pinborg A, Loft A, Laivuori H, Petzold M, et al. The effect of paternal factors on perinatal and paediatric outcomes: a systematic review and meta-analysis. Hum Reprod Update. 2018; 24(3):320–389. PMID: 29471389.
Article10. Peng J, Meng Z, Zhou S, Zhou Y, Wu Y, Wang Q, et al. The non-genetic paternal factors for congenital heart defects: a systematic review and meta-analysis. Clin Cardiol. 2019; 42(7):684–691. PMID: 31073996.
Article11. Nicoletti D, Appel LD, Siedersberger Neto P, Guimarães GW, Zhang L. Maternal smoking during pregnancy and birth defects in children: a systematic review with meta-analysis. Cad Saude Publica. 2014; 30(12):2491–2529. PMID: 26247979.
Article12. Zheng Z, Xie G, Yang T, Qin J. Congenital malformations are associated with secondhand smoke among nonsmoking women: a meta-analysis. Birth. 2019; 46(2):222–233. PMID: 30284325.
Article13. Tanoshima M, Kobayashi T, Tanoshima R, Beyene J, Koren G, Ito S. Risks of congenital malformations in offspring exposed to valproic acid in utero: a systematic review and cumulative meta-analysis. Clin Pharmacol Ther. 2015; 98(4):417–441. PMID: 26044279.14. Gao SY, Wu QJ, Zhang TN, Shen ZQ, Liu CX, Xu X, et al. Fluoxetine and congenital malformations: a systematic review and meta-analysis of cohort studies. Br J Clin Pharmacol. 2017; 83(10):2134–2147. PMID: 28513059.
Article15. Luteijn JM, Brown MJ, Dolk H. Influenza and congenital anomalies: a systematic review and meta-analysis. Hum Reprod. 2014; 29(4):809–823. PMID: 24365800.
Article16. Wen J, Jiang J, Ding C, Dai J, Liu Y, Xia Y, et al. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril. 2012; 97(6):1331–1337.e1-4. PMID: 22480819.17. Zheng Z, Chen L, Yang T, Yu H, Wang H, Qin J. Multiple pregnancies achieved with IVF/ICSI and risk of specific congenital malformations: a meta-analysis of cohort studies. Reprod Biomed Online. 2018; 36(4):472–482. PMID: 29609768.
Article18. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017; 358(1):j4008. PMID: 28935701.
Article19. Chen EK, Zmirou-Navier D, Padilla C, Deguen S. Effects of air pollution on the risk of congenital anomalies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014; 11(8):7642–7668. PMID: 25089772.
Article20. Hall KC, Robinson JC. Association between maternal exposure to pollutant particulate matter 2.5 and congenital heart defects: a systematic review. JBI Database Syst Rev Implement Reports. 2019; 17(8):1695–1716.21. Spinder N, Prins JR, Bergman JE, Smidt N, Kromhout H, Boezen HM, et al. Congenital anomalies in the offspring of occupationally exposed mothers: a systematic review and meta-analysis of studies using expert assessment for occupational exposures. Hum Reprod. 2019; 34(5):903–919. PMID: 30927411.
Article22. Salmasi G, Grady R, Jones J, McDonald SD. Knowledge Synthesis Group. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010; 89(4):423–441. PMID: 20085532.
Article23. Lee LJ, Lupo PJ. Maternal smoking during pregnancy and the risk of congenital heart defects in offspring: a systematic review and metaanalysis. Pediatr Cardiol. 2013; 34(2):398–407. PMID: 22886364.
Article24. Zhang D, Cui H, Zhang L, Huang Y, Zhu J, Li X. Is maternal smoking during pregnancy associated with an increased risk of congenital heart defects among offspring? A systematic review and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2017; 30(6):645–657. PMID: 27126055.
Article25. Zhao L, Chen L, Yang T, Wang L, Wang T, Zhang S, et al. Parental smoking and the risk of congenital heart defects in offspring: an updated meta-analysis of observational studies. Eur J Prev Cardiol. 2020; 27(12):1284–1293. PMID: 30905164.
Article26. Yu C, Wei Y, Tang X, Liu B, Shen L, Long C, et al. Maternal smoking during pregnancy and risk of cryptorchidism: a systematic review and meta-analysis. Eur J Pediatr. 2019; 178(3):287–297. PMID: 30465272.
Article27. Sun J, Chen X, Chen H, Ma Z, Zhou J. Maternal alcohol consumption before and during pregnancy and the risks of congenital heart defects in offspring: a systematic review and meta-analysis. Congenit Heart Dis. 2015; 10(5):E216–E224. PMID: 26032942.
Article28. Wen Z, Yu D, Zhang W, Fan C, Hu L, Feng Y, et al. Association between alcohol consumption during pregnancy and risks of congenital heart defects in offspring: meta-analysis of epidemiological observational studies. Ital J Pediatr. 2016; 42(1):12. PMID: 26843087.
Article29. Bell JC, Raynes-Greenow C, Turner RM, Bower C, Nassar N, O'Leary CM. Maternal alcohol consumption during pregnancy and the risk of orofacial clefts in infants: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2014; 28(4):322–332. PMID: 24800624.
Article30. Ye Z, Wang L, Yang T, Chen L, Wang T, Chen L, et al. Maternal viral infection and risk of fetal congenital heart diseases: a meta-analysis of observational studies. J Am Heart Assoc. 2019; 8(9):e011264. PMID: 30995883.
Article31. Shi QY, Zhang JB, Mi YQ, Song Y, Ma J, Zhang YL. Congenital heart defects and maternal fever: systematic review and meta-analysis. J Perinatol. 2014; 34(9):677–682. PMID: 24811224.
Article32. Balsells M, García-Patterson A, Gich I, Corcoy R. Major congenital malformations in women with gestational diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012; 28(3):252–257. PMID: 22052679.
Article33. Zhao E, Zhang Y, Zeng X, Liu B. Association between maternal diabetes mellitus and the risk of congenital malformations: a meta-analysis of cohort studies. Drug Discov Ther. 2015; 9(4):274–281. PMID: 26370526.
Article34. Simeone RM, Devine OJ, Marcinkevage JA, Gilboa SM, Razzaghi H, Bardenheier BH, et al. Diabetes and congenital heart defects: a systematic review, meta-analysis, and modeling project. Am J Prev Med. 2015; 48(2):195–204. PMID: 25326416.35. Hoang TT, Marengo LK, Mitchell LE, Canfield MA, Agopian AJ. Original findings and updated meta-analysis for the association between maternal diabetes and risk for congenital heart disease phenotypes. Am J Epidemiol. 2017; 186(1):118–128. PMID: 28505225.
Article36. Cai GJ, Sun XX, Zhang L, Hong Q. Association between maternal body mass index and congenital heart defects in offspring: a systematic review. Am J Obstet Gynecol. 2014; 211(2):91–117. PMID: 24631708.
Article37. Zhu Y, Chen Y, Feng Y, Yu D, Mo X. Association between maternal body mass index and congenital heart defects in infants: a meta-analysis. Congenit Heart Dis. 2018; 13(2):271–281. PMID: 29363266.
Article38. Davenport MH, Yoo C, Mottola MF, Poitras VJ, Jaramillo Garcia A, Gray CE, et al. Effects of prenatal exercise on incidence of congenital anomalies and hyperthermia: a systematic review and meta-analysis. Br J Sports Med. 2019; 53(2):116–123. PMID: 30337347.
Article39. Yu HF, Chen HS, Rao DP, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2016; 95(51):e4863. PMID: 28002314.40. Feng Y, Yu D, Chen T, Liu J, Tong X, Yang L, et al. Maternal parity and the risk of congenital heart defects in offspring: a dose-response meta-analysis of epidemiological observational studies. PLoS One. 2014; 9(10):e108944. PMID: 25295723.
Article41. Yakoob MY, Bateman BT, Ho E, Hernandez-Diaz S, Franklin JM, Goodman JE, et al. The risk of congenital malformations associated with exposure to β-blockers early in pregnancy: a meta-analysis. Hypertension. 2013; 62(2):375–381. PMID: 23753416.42. Grigoriadis S, Vonderporten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, et al. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta-analysis. BMJ. 2014; 348(1):f6932. PMID: 24429387.
Article43. Wang S, Yang L, Wang L, Gao L, Xu B, Xiong Y. Selective serotonin reuptake inhibitors (SSRIs) and the risk of congenital heart defects: a meta-analysis of prospective cohort studies. J Am Heart Assoc. 2015; 4(5):e001681. PMID: 25991012.
Article44. Feng Y, Wang S, Chen R, Tong X, Wu Z, Mo X. Maternal folic acid supplementation and the risk of congenital heart defects in offspring: a meta-analysis of epidemiological observational studies. Sci Rep. 2015; 5(1):8506. PMID: 25687545.
Article45. Xu A, Cao X, Lu Y, Li H, Zhu Q, Chen X, et al. A meta-analysis of the relationship between maternal folic acid supplementation and the risk of congenital heart defects. Int Heart J. 2016; 57(6):725–728. PMID: 27829639.
Article46. Li ZX, Gao ZL, Wang JN, Guo QH. Maternal coffee consumption during pregnancy and neural tube defects in offspring: a meta-analysis. Fetal Pediatr Pathol. 2016; 35(1):1–9. PMID: 26720182.
Article47. Massaro PA, MacLellan DL, Anderson PA, Romao RL. Does intracytoplasmic sperm injection pose an increased risk of genitourinary congenital malformations in offspring compared to in vitro fertilization? A systematic review and meta-analysis. J Urol. 2015; 193(5):Suppl. 1837–1842. PMID: 25813561.48. Qin J, Sheng X, Wang H, Liang D, Tan H, Xia J. Assisted reproductive technology and risk of congenital malformations: a meta-analysis based on cohort studies. Arch Gynecol Obstet. 2015; 292(4):777–798. PMID: 25877221.
Article49. Qin J, Liu X, Sheng X, Wang H, Gao S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril. 2016; 105(1):73–85.e1. PMID: 26453266.
Article50. Lacamara C, Ortega C, Villa S, Pommer R, Schwarze JE. Are children born from singleton pregnancies conceived by ICSI at increased risk for congenital malformations when compared to children conceived naturally? A systematic review and meta-analysis. JBRA Assist Reprod. 2017; 21(3):251–259. PMID: 28837036.
Article51. Yu D, Feng Y, Yang L, Da M, Fan C, Wang S, et al. Maternal socioeconomic status and the risk of congenital heart defects in offspring: a meta-analysis of 33 studies. PLoS One. 2014; 9(10):e111056. PMID: 25347676.
Article52. Deguen S, Kihal W, Jeanjean M, Padilla C, Zmirou-Navier D. Neighborhood deprivation and risk of congenital heart defects, neural tube defects and orofacial clefts: a systematic review and meta-analysis. PLoS One. 2016; 11(10):e0159039. PMID: 27783616.
Article53. Daliri S, Safarpour H, Bazyar J, Sayehmiri K, Karimi A, Anvary R. The relationship between some neonatal and maternal factors during pregnancy with the prevalence of congenital malformations in Iran: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2019; 32(21):3666–3674. PMID: 29739244.
Article54. Chen KH, Chen LL, Li WG, Fang Y, Huang GY. Maternal MTHFR C677T polymorphism and congenital heart defect risk in the Chinese Han population: a meta-analysis. Genet Mol Res. 2013; 12(4):6212–6219. PMID: 24338416.55. Zhang R, Huo C, Wang X, Dang B, Mu Y, Wang Y. Two common MTHFR gene polymorphisms (C677T and A1298C) and fetal congenital heart disease risk: an updated meta-analysis with trial sequential analysis. Cell Physiol Biochem. 2018; 45(6):2483–2496. PMID: 29554656.56. Yuan Y, Yu X, Niu F, Lu N. Genetic polymorphism of methylenetetrahydrofolate reductase as a potential risk factor for congenital heart disease: a meta-analysis in Chinese pediatric population. Medicine (Baltimore). 2017; 96(23):e7057. PMID: 28591039.57. Yang HL, Yang YL, Yu CH, Shiao SP. Meta-prediction of MTHFR gene polymorphism and air pollution on the risks of congenital heart defects worldwide: a transgenerational analysis. Int J Environ Res Public Health. 2018; 15(8):E1660. PMID: 30081597.58. Xuan C, Li H, Zhao JX, Wang HW, Wang Y, Ning CP, et al. Association between MTHFR polymorphisms and congenital heart disease: a meta-analysis based on 9,329 cases and 15,076 controls. Sci Rep. 2014; 4(1):7311. PMID: 25472587.
Article59. Wang W, Hou Z, Wang C, Wei C, Li Y, Jiang L. Association between 5, 10-methylenetetrahydrofolate reductase (MTHFR) polymorphisms and congenital heart disease: a meta-analysis. Meta Gene. 2013; 1(1):109–125. PMID: 25606381.60. Yin M, Dong L, Zheng J, Zhang H, Liu J, Xu Z. Meta analysis of the association between MTHFR C677T polymorphism and the risk of congenital heart defects. Ann Hum Genet. 2012; 76(1):9–16. PMID: 22175539.61. van Beynum IM, den Heijer M, Blom HJ, Kapusta L. The MTHFR 677C->T polymorphism and the risk of congenital heart defects: a literature review and meta-analysis. QJM. 2007; 100(12):743–753. PMID: 17965089.62. Nie Y, Gu H, Gong J, Wang J, Gong D, Cong X, et al. Methylenetetrahydrofolate reductase C677T polymorphism and congenital heart disease: a meta-analysis. Clin Chem Lab Med. 2011; 49(12):2101–2108. PMID: 21793799.
Article63. Li Z, Jun Y, Zhong-Bao R, Jie L, Jian-Ming L. Association between MTHFR C677T polymorphism and congenital heart disease. A family-based meta-analysis. Herz. 2015; 40(Suppl 2):160–167. PMID: 25256053.64. Verkleij-Hagoort A, Bliek J, Sayed-Tabatabaei F, Ursem N, Steegers E, Steegers-Theunissen R. Hyperhomocysteinemia and MTHFR polymorphisms in association with orofacial clefts and congenital heart defects: a meta-analysis. Am J Med Genet A. 2007; 143A(9):952–960. PMID: 17431894.65. Yu D, Zhuang Z, Wen Z, Zang X, Mo X. MTHFR A1298C polymorphisms reduce the risk of congenital heart defects: a meta-analysis from 16 case-control studies. Ital J Pediatr. 2017; 43(1):108. PMID: 29202788.
Article66. Cai B, Zhang T, Zhong R, Zou L, Zhu B, Chen W, et al. Genetic variant in MTRR, but not MTR, is associated with risk of congenital heart disease: an integrated meta-analysis. PLoS One. 2014; 9(3):e89609. PMID: 24595101.67. Yu D, Yang L, Shen S, Fan C, Zhang W, Mo X. Association between methionine synthase reductase A66G polymorphism and the risk of congenital heart defects: evidence from eight case-control studies. Pediatr Cardiol. 2014; 35(7):1091–1098. PMID: 24913415.
Article68. Zhang Y, Ai F, Zheng J, Peng B. Associations of GATA4 genetic mutations with the risk of congenital heart disease: a meta-analysis. Medicine (Baltimore). 2017; 96(18):e6857. PMID: 28471988.69. Wang Z, Zou L, Zhong R, Zhu B, Chen W, Shen N, et al. Associations between two genetic variants in NKX2-5 and risk of congenital heart disease in Chinese population: a meta-analysis. PLoS One. 2013; 8(8):e70979. PMID: 23936479.70. Zhang K, Li Y, Mao Y, Ma M. Steroid 5-alpha-reductase type 2 (SRD5A2) gene V89L polymorphism and hypospadias risk: a meta-analysis. J Pediatr Urol. 2017; 13(6):630.e1–630.e9. PMID: 28713005.71. Xu X, Zheng J, Liao Q, Zhu H, Xie H, Shi H, et al. Meta-analyses of 4 CFTR variants associated with the risk of the congenital bilateral absence of the vas deferens. J Clin Bioinforma. 2014; 4(1):11. PMID: 25170420.72. Huang E, Cheng H, Xu M, Shu S, Tang S. Association between single-nucleotide polymorphisms on chromosome 1p22 and 20q12 and nonsyndromic cleft lip with or without cleft palate: new data in Han Chinese and meta-analysis. Birth Defects Res A Clin Mol Teratol. 2012; 94(6):469–476. PMID: 22522387.
Article73. Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008; 151(2):362–367. PMID: 17646040.
Article74. Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006; 114(11):1636–1642. PMID: 17107846.
Article75. Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect. 2008; 116(6):791–798. PMID: 18560536.
Article76. Streissguth AP, Landesman-Dwyer S, Martin JC, Smith DW. Teratogenic effects of alcohol in humans and laboratory animals. Science. 1980; 209(4454):353–361. PMID: 6992275.
Article77. Mamluk L, Edwards HB, Savović J, Leach V, Jones T, Moore TH, et al. Low alcohol consumption and pregnancy and childhood outcomes: time to change guidelines indicating apparently ‘safe’ levels of alcohol during pregnancy? A systematic review and meta-analyses. BMJ Open. 2017; 7(7):e015410.
Article78. Popova S, Lange S, Shield K, Mihic A, Chudley AE, Mukherjee RA, et al. Comorbidity of fetal alcohol spectrum disorder: a systematic review and meta-analysis. Lancet. 2016; 387(10022):978–987. PMID: 26777270.
Article79. Moretti ME, Bar-Oz B, Fried S, Koren G. Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology. 2005; 16(2):216–219. PMID: 15703536.80. Gu J, Xie Z, Gao Z, Liu J, Korteweg C, Ye J, et al. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet. 2007; 370(9593):1137–1145. PMID: 17905166.
Article81. Styrud J, Thunberg L, Nybacka O, Eriksson UJ. Correlations between maternal metabolism and deranged development in the offspring of normal and diabetic rats. Pediatr Res. 1995; 37(3):343–353. PMID: 7784144.
Article82. Roest PA, van Iperen L, Vis S, Wisse LJ, Poelmann RE, Steegers-Theunissen RP, et al. Exposure of neural crest cells to elevated glucose leads to congenital heart defects, an effect that can be prevented by N-acetylcysteine. Birth Defects Res A Clin Mol Teratol. 2007; 79(3):231–235. PMID: 17183584.
Article83. Epstein JA, Li J, Lang D, Chen F, Brown CB, Jin F, et al. Migration of cardiac neural crest cells in Splotch embryos. Development. 2000; 127(9):1869–1878. PMID: 10751175.
Article84. Mojtabai R. Body mass index and serum folate in childbearing age women. Eur J Epidemiol. 2004; 19(11):1029–1036. PMID: 15648596.
Article85. Caton AR, Bell EM, Druschel CM, Werler MM, Lin AE, Browne ML, et al. Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertension. 2009; 54(1):63–70. PMID: 19433779.
Article86. Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ. 2011; 343(1):d5931. PMID: 22010128.
Article87. Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. An overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997; 32(Suppl 1):1–21.88. Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry. 2003; 160(5):993–996. PMID: 12727706.
Article89. Sadler TW. Selective serotonin reuptake inhibitors (SSRIs) and heart defects: potential mechanisms for the observed associations. Reprod Toxicol. 2011; 32(4):484–489. PMID: 21963886.
Article90. Sari Y, Zhou FC. Serotonin and its transporter on proliferation of fetal heart cells. Int J Dev Neurosci. 2003; 21(8):417–424. PMID: 14659992.
Article91. Yavarone MS, Shuey DL, Tamir H, Sadler TW, Lauder JM. Serotonin and cardiac morphogenesis in the mouse embryo. Teratology. 1993; 47(6):573–584. PMID: 8367830.
Article92. Ericson A, Källén B. Congenital malformations in infants born after IVF: a population-based study. Hum Reprod. 2001; 16(3):504–509. PMID: 11228220.
Article93. Lambert RD. Safety issues in assisted reproductive technology: aetiology of health problems in singleton ART babies. Hum Reprod. 2003; 18(10):1987–1991. PMID: 14507811.94. Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012; 366(19):1803–1813. PMID: 22559061.
Article95. Retzloff MG, Hornstein MD. Is intracytoplasmic sperm injection safe? Fertil Steril. 2003; 80(4):851–859. PMID: 14556800.
Article96. Wen SW, Leader A, White RR, Léveillé MC, Wilkie V, Zhou J, et al. A comprehensive assessment of outcomes in pregnancies conceived by in vitro fertilization/intracytoplasmic sperm injection. Eur J Obstet Gynecol Reprod Biol. 2010; 150(2):160–165. PMID: 20207067.
Article97. Biselli PM, Guerzoni AR, de Godoy MF, Eberlin MN, Haddad R, Carvalho VM, et al. Genetic polymorphisms involved in folate metabolism and concentrations of methylmalonic acid and folate on plasma homocysteine and risk of coronary artery disease. J Thromb Thrombolysis. 2010; 29(1):32–40. PMID: 19283448.
Article98. Brouns R, Ursem N, Lindemans J, Hop W, Pluijm S, Steegers E, et al. Polymorphisms in genes related to folate and cobalamin metabolism and the associations with complex birth defects. Prenat Diagn. 2008; 28(6):485–493. PMID: 18435414.
Article99. Kluijtmans LA, Young IS, Boreham CA, Murray L, McMaster D, McNulty H, et al. Genetic and nutritional factors contributing to hyperhomocysteinemia in young adults. Blood. 2003; 101(7):2483–2488. PMID: 12642343.
Article100. Gur S. Guilt feelings in parents of children with congenital defects. Reconstr Surg Traumatol. 1974; 14(0):157–160. PMID: 4841550.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neonatal risk factors associated with attention-deficit/hyperactivity disorder: an umbrella review
- Introduction to systematic review and meta-analysis
- Neonatal risk factors associated with autism spectrum disorders: an umbrella review
- Intracranial Pressure Monitoring in Patients With Traumatic Brain Injury: An Umbrella Review of Systematic Review and Meta-Analysis
- Introduction to Umbrella Reviews as a Useful Evidence-Based Practice