Allergy Asthma Immunol Res.
2017 May;9(3):220-228. 10.4168/aair.2017.9.3.220.
Efficacy of Sublingual Immunotherapy for House Dust Mite-Induced Allergic Rhinitis: A Meta-Analysis of Randomized Controlled Trials
- Affiliations
-
- 1Department of Otolaryngology, the Second Affiliated Hospital & Yuying Children's Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China. wzbobei@hotmail.com
- KMID: 2371722
- DOI: http://doi.org/10.4168/aair.2017.9.3.220
Abstract
- PURPOSE
Allergic rhinitis (AR) has become a global issue for a large part of the general population. Sublingual immunotherapy (SLIT) has been used extensively to treat persistent allergic rhinitis (PAR). Although systematic reviews have confirmed the effectiveness of SLIT for the treatment of AR, a considerable number of studies using extracts of house dust mites (HDMs) for immunotherapy found no consensus on basic treatment parameters and questioned the efficacy of SLIT.
METHODS
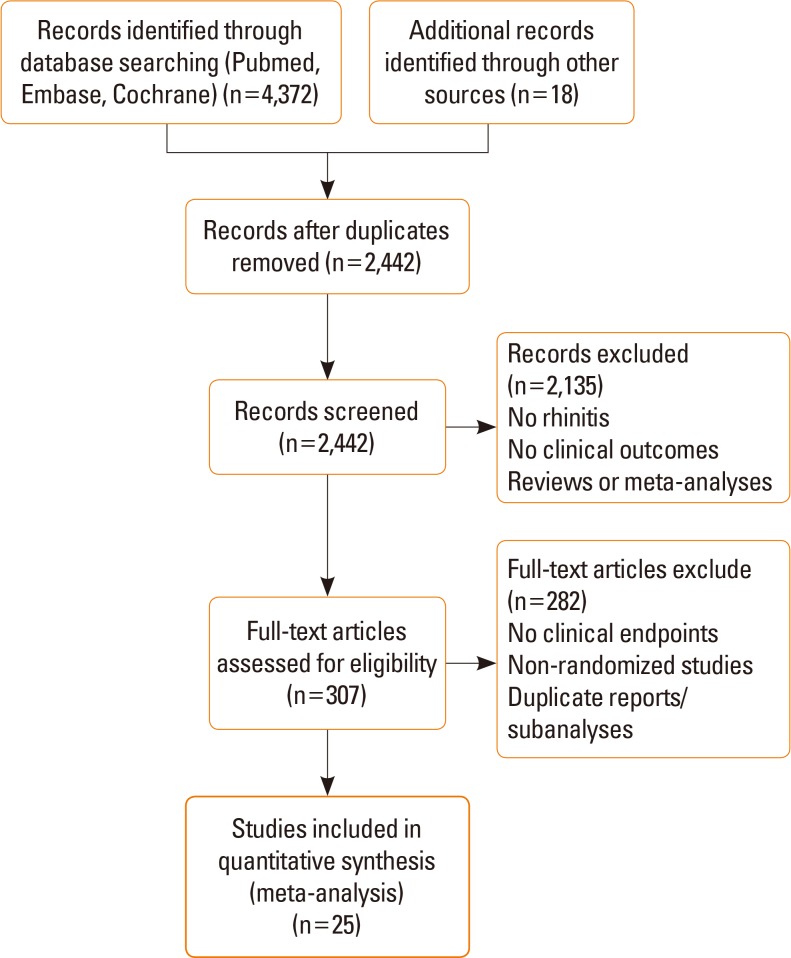
In this study, we evaluated SLIT for PAR by a meta-analysis of randomized controlled trials (RCTs). Medline, Embase, and Cochrane Library database searches were performed for RCTs on the treatment of PAR by SLIT that assessed clinical outcomes related to efficacy through May 2016. Descriptive and quantitative information was abstracted. An analysis was performed with standardized mean differences (SMDs) under a fixed or random effects model. Subgroup analyses were performed. Heterogeneity was assessed using the I2 metric.
RESULTS
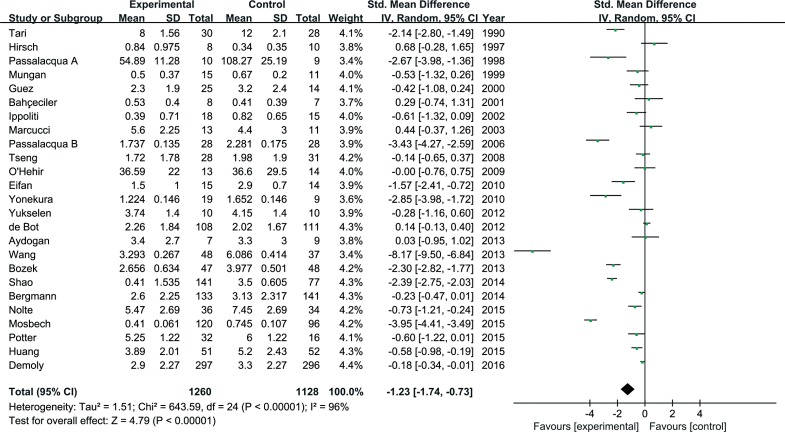
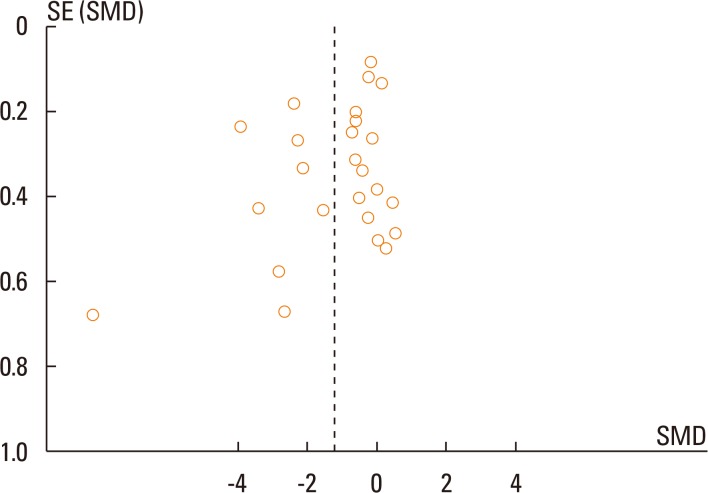
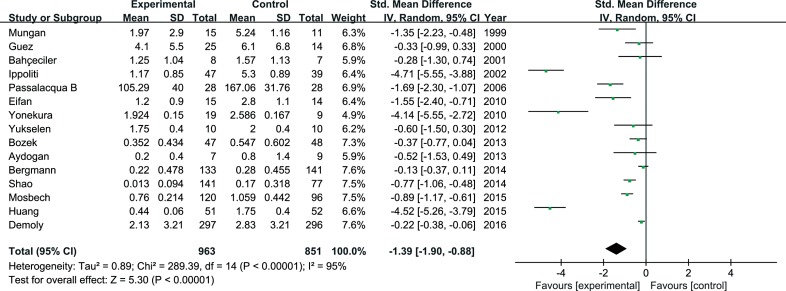
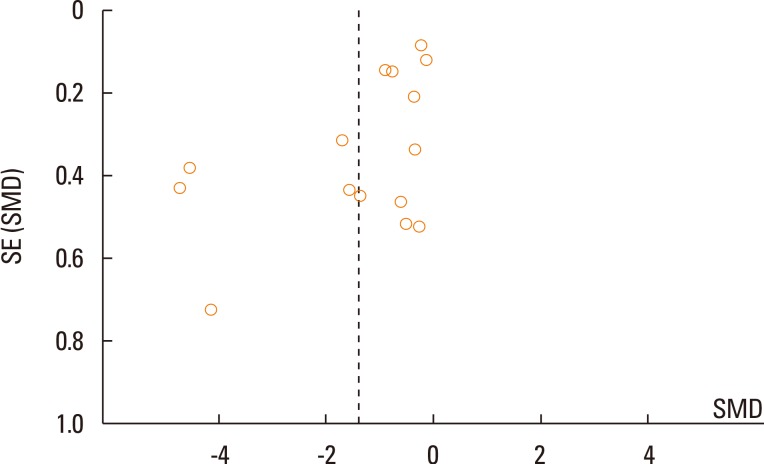
In total, 25 studies were eligible for inclusion in the meta-analysis for symptom scores and 15 studies for medication scores. SLIT was significantly different from the controls for symptom scores (SMD=1.23; 95% confidence interval [CI]=1.74 to 0.73; P<0.001). For medication scores, significant differences for SLIT were also observed versus the controls (SMD=-1.39; 95% CI=-1.90 to -0.88; P<0.001).
CONCLUSIONS
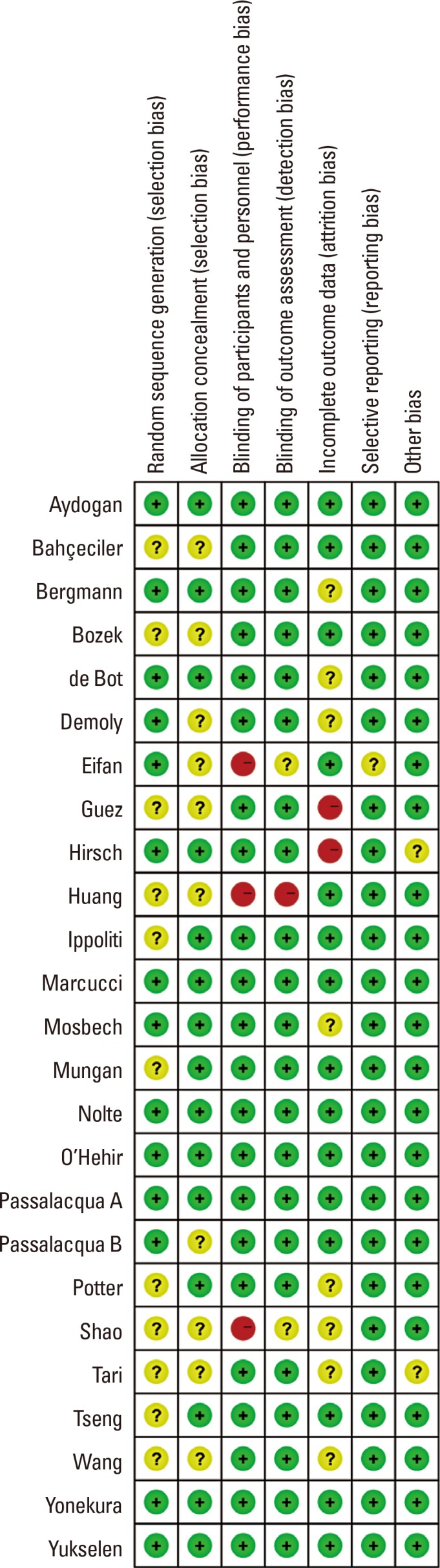
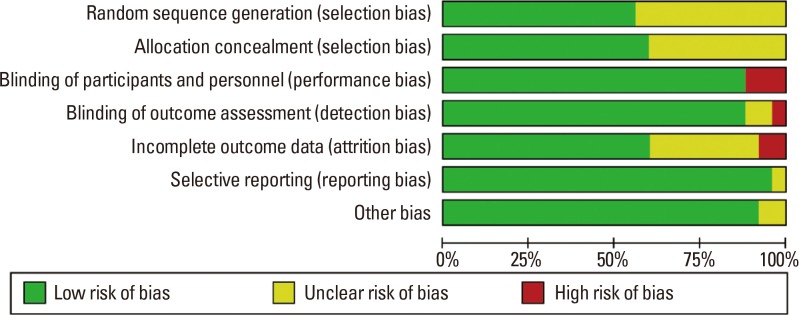
Our meta-analysis indicates that SLIT provided significant symptom relief and reduced the need for medications in PAR. In this study, significant evidence was obtained despite heterogeneity with regard to the use of mite extract. Specifically, the mite extract used was provided by the patients with PAR. Furthermore, to confirm both the objective outcomes and the effective doses of HDM allergen extracts, experimental data should be obtained from large high-quality population-based studies.
MeSH Terms
Figure
Cited by 2 articles
-
Efficacy and Safety of Sublingual Immunotherapy in Elderly Rhinitis Patients Sensitized to House Dust Mites
Ji Hye Kim, Ji Ho Lee, Young-Min Ye, Jae-Hyun Lee, Jung Won Park, Gyu-Young Hur, Joo-Hee Kim, Hyn-Young Lee, Yoo Seob Shin, Eun-Mi Yang, Hae-Sim Park
Allergy Asthma Immunol Res. 2018;10(6):675-685. doi: 10.4168/aair.2018.10.6.675.Efficacy of Allergen Immunotherapy for Allergic Asthma in Real World Practice
Hyo-In Rhyou, Young-Hee Nam
Allergy Asthma Immunol Res. 2020;12(1):99-109. doi: 10.4168/aair.2020.12.1.99.
Reference
-
1. Salo PM, Calatroni A, Gergen PJ, Hoppin JA, Sever ML, Jaramillo R, et al. Allergy-related outcomes in relation to serum IgE: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2011; 127:1226–1235.e7. PMID: 21320720.
Article2. Bousquet PJ, Chinn S, Janson C, Kogevinas M, Burney P, Jarvis D, et al. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy. 2007; 62:301–309. PMID: 17298348.
Article3. Linneberg A, Henrik Nielsen N, Frølund L, Madsen F, Dirksen A, Jørgensen T, et al. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2002; 57:1048–1052. PMID: 12359002.
Article4. Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, et al. Respiratory allergy caused by house dust mites: What do we really know? J Allergy Clin Immunol. 2015; 136:38–48. PMID: 25457152.
Article5. Miyazawa H, Sakaguchi M, Inouye S, Ikeda K, Honbo Y, Yasueda H, et al. Seasonal changes in mite allergen (Der I and Der II) concentrations in Japanese homes. Ann Allergy Asthma Immunol. 1996; 76:170–174. PMID: 8595537.
Article6. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011; 106:S12–S16. PMID: 21277528.
Article7. Yoo KH, Ahn HR, Park JK, Kim JW, Nam GH, Hong SK, et al. Burden of respiratory disease in Korea: an observational study on allergic rhinitis, asthma, COPD, and rhinosinusitis. Allergy Asthma Immunol Res. 2016; 8:527–534. PMID: 27582404.
Article8. Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010; 126:466–476. PMID: 20816182.9. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.e3. PMID: 23498595.10. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016; 8:191–197. PMID: 26922928.
Article11. Morjaria JB, Caruso M, Rosalia E, Russo C, Polosa R. Preventing progression of allergic rhinitis to asthma. Curr Allergy Asthma Rep. 2014; 14:412. PMID: 24408536.
Article12. Dong X, Huang N, Li W, Hu L, Wang X, Wang Y, et al. Systemic reactions to dust mite subcutaneous immunotherapy: a 3-year follow-up study. Allergy Asthma Immunol Res. 2016; 8:421–427. PMID: 27334780.
Article13. Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization position paper 2009. Allergy. 2009; 64(Suppl 91):1–59.14. Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013; 132:1322–1336. PMID: 24139829.15. Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ. 1999; 318:593–596. PMID: 10037645.
Article16. Jørgensen AW, Hilden J, Gøtzsche PC. Cochrane reviews compared with industry supported meta-analyses and other meta-analyses of the same drugs: systematic review. BMJ. 2006; 333:782. PMID: 17028106.
Article17. Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Di Lorenzo G. Efficacy of grass pollen allergen sublingual immunotherapy tablets for seasonal allergic rhinoconjunctivitis: a systematic review and meta-analysis. JAMA Intern Med. 2015; 175:1301–1309. PMID: 26120825.18. Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010; CD002893. PMID: 21154351.
Article19. Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005; 60:4–12. PMID: 15575924.
Article20. Compalati E, Passalacqua G, Bonini M, Canonica GW. The efficacy of sublingual immunotherapy for house dust mites respiratory allergy: results of a GA2LEN meta-analysis. Allergy. 2009; 64:1570–1579. PMID: 19796205.21. Aydogan M, Eifan AO, Keles S, Akkoc T, Nursoy MA, Bahceciler NN, et al. Sublingual immunotherapy in children with allergic rhinoconjunctivitis mono-sensitized to house-dust-mites: a double-blind-placebo-controlled randomised trial. Respir Med. 2013; 107:1322–1329. PMID: 23886432.
Article22. Bahçeciler NN, Işik U, Barlan IB, Başaran MM. Efficacy of sublingual immunotherapy in children with asthma and rhinitis: a double-blind, placebo-controlled study. Pediatr Pulmonol. 2001; 32:49–55. PMID: 11416876.
Article23. Bergmann KC, Demoly P, Worm M, Fokkens WJ, Carrillo T, Tabar AI, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014; 133:1608–1614.e6. PMID: 24388010.
Article24. Bozek A, Ignasiak B, Filipowska B, Jarzab J. House dust mite sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with allergic rhinitis. Clin Exp Allergy. 2013; 43:242–248. PMID: 23331565.
Article25. de Bot CM, Moed H, Berger MY, Röder E, Hop WC, de Groot H, et al. Sublingual immunotherapy not effective in house dust mite-allergic children in primary care. Pediatr Allergy Immunol. 2012; 23:150–158. PMID: 22017365.
Article26. Demoly P, Emminger W, Rehm D, Backer V, Tommerup L, Kleine-Tebbe J. Effective treatment of house dust mite-induced allergic rhinitis with 2 doses of the SQ HDM SLIT-tablet: results from a randomized, double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2016; 137:444–451.e8. PMID: 26292778.
Article27. Eifan AO, Akkoc T, Yildiz A, Keles S, Ozdemir C, Bahceciler NN, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. 2010; 40:922–932. PMID: 20100188.
Article28. Guez S, Vatrinet C, Fadel R, André C. House-dust-mite sublingual-swallow immunotherapy (SLIT) in perennial rhinitis: a double-blind, placebo-controlled study. Allergy. 2000; 55:369–375. PMID: 10782522.
Article29. Hirsch T, Sähn M, Leupold W. Double-blind placebo-controlled study of sublingual immunotherapy with house dust mite extract (D.pt.) in children. Pediatr Allergy Immunol. 1997; 8:21–27.30. Huang S, Xie X, Chen Y, Wu L, Li R, Shen F, et al. Study on the efficacy and safety of sublingual immunotherapy with standardized Dermatophagoides farinae drops for allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015; 29:618–621. PMID: 26201187.31. Ippoliti F, De Santis W, Volterrani A, Lenti L, Canitano N, Lucarelli S, et al. Immunomodulation during sublingual therapy in allergic children. Pediatr Allergy Immunol. 2003; 14:216–221. PMID: 12787302.
Article32. Marcucci F, Sensi L, Frati F, Bernardini R, Novembre E, Barbato A, et al. Effects on inflammation parameters of a double-blind, placebo controlled one-year course of SLIT in children monosensitized to mites. Allergy. 2003; 58:657–662. PMID: 12823127.
Article33. Mosbech H, Canonica GW, Backer V, de Blay F, Klimek L, Broge L, et al. SQ house dust mite sublingually administered immunotherapy tablet (ALK) improves allergic rhinitis in patients with house dust mite allergic asthma and rhinitis symptoms. Ann Allergy Asthma Immunol. 2015; 114:134–140. PMID: 25624131.
Article34. Mungan D, Misirligil Z, Gürbüz L. Comparison of the efficacy of subcutaneous and sublingual immunotherapy in mite-sensitive patients with rhinitis and asthma--a placebo controlled study. Ann Allergy Asthma Immunol. 1999; 82:485–490. PMID: 10353581.
Article35. Nolte H, Maloney J, Nelson HS, Bernstein DI, Lu S, Li Z, et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J Allergy Clin Immunol. 2015; 135:1494–1501.e6. PMID: 25636947.
Article36. O'Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009; 180:936–947. PMID: 19696440.37. Passalacqua G, Albano M, Fregonese L, Riccio A, Pronzato C, Mela GS, et al. Randomised controlled trial of local allergoid immunotherapy on allergic inflammation in mite-induced rhinoconjunctivitis. Lancet. 1998; 351:629–632. PMID: 9500318.
Article38. Passalacqua G, Pasquali M, Ariano R, Lombardi C, Giardini A, Baiardini I, et al. Randomized double-blind controlled study with sublingual carbamylated allergoid immunotherapy in mild rhinitis due to mites. Allergy. 2006; 61:849–854. PMID: 16792583.
Article39. Potter PC, Baker S, Fenemore B, Nurse B. Clinical and cytokine responses to house dust mite sublingual immunotherapy. Ann Allergy Asthma Immunol. 2015; 114:327–334. PMID: 25661658.
Article40. Shao J, Cui YX, Zheng YF, Peng HF, Zheng ZL, Chen JY, et al. Efficacy and safety of sublingual immunotherapy in children aged 3–13 years with allergic rhinitis. Am J Rhinol Allergy. 2014; 28:131–139. PMID: 24717951.
Article41. Tari MG, Mancino M, Monti G. Efficacy of sublingual immunotherapy in patients with rhinitis and asthma due to house dust mite. A double-blind study. Allergol Immunopathol (Madr). 1990; 18:277–284. PMID: 2097894.42. Tseng SH, Fu LS, Nong BR, Weng JD, Shyur SD. Changes in serum specific IgG4 and IgG4/IgE ratio in mite-sensitized Taiwanese children with allergic rhinitis receiving short-term sublingual-swallow immunotherapy: a multicenter, randomized, placebo-controlled trial. Asian Pac J Allergy Immunol. 2008; 26:105–112. PMID: 19054928.43. Wang DH, Chen L, Cheng L, Li KN, Yuan H, Lu JH, et al. Fast onset of action of sublingual immunotherapy in house dust mite-induced allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial. Laryngoscope. 2013; 123:1334–1340. PMID: 23616386.
Article44. Yonekura S, Okamoto Y, Sakurai D, Horiguchi S, Hanazawa T, Nakano A, et al. Sublingual immunotherapy with house dust extract for house dust-mite allergic rhinitis in children. Allergol Int. 2010; 59:381–388. PMID: 20864799.
Article45. Yukselen A, Kendirli SG, Yilmaz M, Altintas DU, Karakoc GB. Effect of one-year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo-controlled, double-blind, double-dummy study. Int Arch Allergy Immunol. 2012; 157:288–298. PMID: 22041501.
Article46. Nelson HS. Update on house dust mite immunotherapy: are more studies needed? Curr Opin Allergy Clin Immunol. 2014; 14:542–548. PMID: 25115684.47. Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006; 117:1021–1035. PMID: 16675328.
Article48. Klimek L, Mosbech H, Zieglmayer P, Rehm D, Stage BS, Demoly P. SQ house dust mite (HDM) SLIT-tablet provides clinical improvement in HDM-induced allergic rhinitis. Expert Rev Clin Immunol. 2016; 12:369–377. PMID: 26788764.
Article49. Lin SY, Erekosima N, Kim JM, Ramanathan M, Suarez-Cuervo C, Chelladurai Y, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA. 2013; 309:1278–1288. PMID: 23532243.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update of Sublingual Immunotherapy for Allergic Rhinitis
- Effect on quality of life of the mixed house dust mite/weed pollen extract immunotherapy
- Head-to-head comparison between subcutaneous and sublingual immunotherapy in perennial allergic rhinitis: A systematic review and meta-analysis
- Outcome of Sublingual Immunotherapy in Patients With Allergic Rhinitis Sensitive to House Dust Mites
- Distribution of House Dust Mites in the Bedroom of Patients with Allergic Rhinitis in Pusan Area