Ann Lab Med.
2015 Sep;35(5):487-493. 10.3343/alm.2015.35.5.487.
Detection of First-Line Anti-Tuberculosis Drug Resistance Mutations by Allele-Specific Primer Extension on a Microsphere-Based Platform
- Affiliations
-
- 1Korean Institute of Tuberculosis, Cheongju, Korea. seung6992@hanmail.net
- 2Genes Laboratories, Seongnam, Korea.
- 3Department of Pathology, Semyung University, Jecheon, Korea.
- KMID: 2369763
- DOI: http://doi.org/10.3343/alm.2015.35.5.487
Abstract
- BACKGROUND
Resistance of Mycobacterium tuberculosis to anti-tuberculosis (TB) drugs is almost exclusively due to spontaneous chromosomal mutations in target genes. Rapid detection of drug resistance to both first- and second-line anti-TB drugs has become a key component of TB control programs. Technologies that allow rapid, cost-effective, and high-throughput detection of specific nucleic acid sequences are needed. This study was to develop a high-throughput assay based on allele-specific primer extension (ASPE) and MagPlex-TAG microspheres to detect anti-TB drug resistance mutations.
METHODS
DNA samples from 357 M. tuberculosis clinical isolates and H37Rv were amplified by multiplex PCR using four primer sets, followed by multiplex ASPE using 23 TAG-ASPE primers. The products were sorted on the TAG-ASPE array and detected by using the Luminex xMAP system. Genotypes were also determined by sequencing.
RESULTS
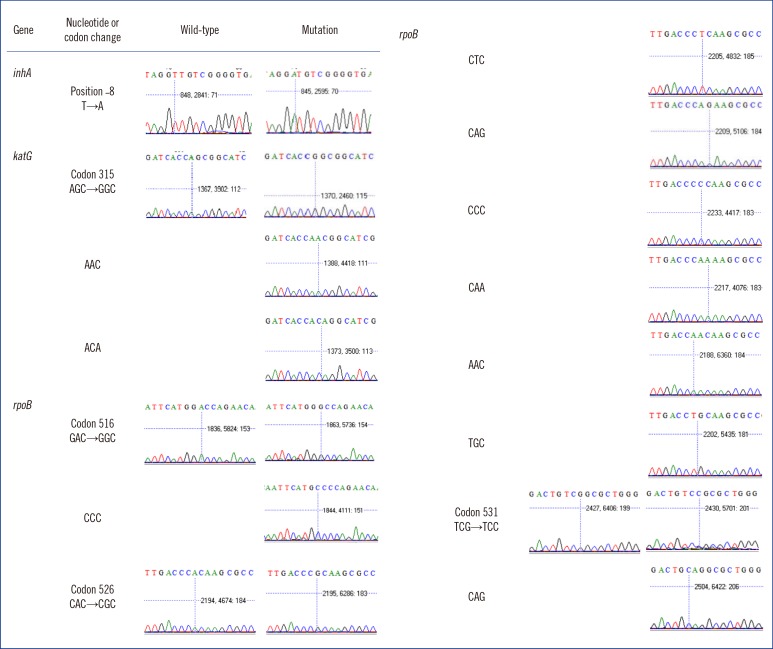
Genetic drug susceptibility typing by the TAG-ASPE method was 100% concordant with those obtained by sequencing. Compared with phenotypic drug susceptibility testing (DST) as a reference method, the sensitivity and specificity of the TAG-ASPE method were 83% (95% confidence interval [CI], 79-88%) and 97% (95% CI, 90-100%) for isoniazid. For rifampin testing, the sensitivity and specificity were 90% (95% CI, 86-93%) and 100% (95% CI, 99-100%). Also, the sensitivity and specificity were 58% (95% CI, 51-65%) and 86% (95% CI, 79-93%) for ethambutol.
CONCLUSIONS
This study demonstrated the TAG-ASPE method is suitable for highly reproducible, cost-effective, and high-throughput clinical genotyping applications.
MeSH Terms
Figure
Cited by 1 articles
-
Molecular Diagnosis of Tuberculosis
Fariz Nurwidya, Diah Handayani, Erlina Burhan, Faisal Yunus
Chonnam Med J. 2018;54(1):1-9. doi: 10.4068/cmj.2018.54.1.1.
Reference
-
1. World Health Organization. Global tuberculosis report 2013. Geneva: World Health Organization Press;2013. Available from http://www.who.int/tb/publications/global_report/en/.2. World Health Organization. Anti-tuberculosis drug resistance in the world. Report no. 4. WHO/HTM/TB/2008.394. Geneva: World Health Organization Press;2008. Available from http://www.who.int/tb/publications/2008/drs_report4_26feb08.pdf.3. Zignol M, Hosseini MS, Wright A, Weezenbeek CL, Nunn P, Watt CJ, et al. Global incidence of multidrug-resistant tuberculosis. J Infect Dis. 2006; 194:479–485. PMID: 16845631.
Article4. Kapur V, Li LL, Hamrick MR, Plikaytis BB, Shinnick TM, Telenti A, et al. Rapid Mycobacterium species assignment and unambiguous identification of mutations associated with antimicrobial resistance in Mycobacterium tuberculosis by automated DNA sequencing. Arch Pathol Lab Med. 1995; 119:131–138. PMID: 7848059.5. Kapur V, Li LL, Iordanescu S, Hamrick MR, Wanger A, Kreiswirth BN, et al. Characterization by automated DNA sequencing of mutations in the gene (rpoB) encoding the RNA polymerase beta subunit in rifampin-resistant Mycobacterium tuberculosis strains from New York City and Texas. J Clin Microbiol. 1994; 32:1095–1098. PMID: 8027320.6. Moghazeh SL, Pan X, Arain T, Stover CK, Musser JM, Kreiswirth BN. Comparative antimycobacterial activities of rifampin, rifapentine, and KRN-1648 against a collection of rifampin-resistant Mycobacterium tuberculosis isolates with known rpoB mutations. Antimicrob Agents Chemother. 1996; 40:2655–2657. PMID: 8913484.7. Musser JM. Antimicrobial agent resistance in mycobacteria: molecular genetic insight. Clin Microbiol Rev. 1995; 8:496–514. PMID: 8665467.8. Musser JM, Kapur V, Williams DL, Kreiswirth BN, van Soolingen D, van Embden JD. Characterization of the catalase-peroxidase gene (katG) and inhA locus in isoniazid-resistant and -susceptible strains of Mycobacterium tuberculosis by automated DNA sequencing: restricted array of mutations associated with drug resistance. J Infect Dis. 1996; 173:196–202. PMID: 8537659.9. Telenti A, Imboden P, Marchesi F, Schmidheini T, Bodmer T. Direct, automated detection of rifampin-resis tant Mycobacterium tuberculosis by polymerase chain reaction and single-strand conformation polymorphism analysis. Antimicrob Agents Chemother. 1993; 37:2054–2058. PMID: 8257122.10. Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992; 358:591–593. PMID: 1501713.11. Ramaswamy SV, Amin AG, Göksel S, Stager CE, Dou SJ, El Sahly H, et al. Molecular genetic analysis of nucleotide polymorphisms associated with ethambutol resistance in human isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2000; 44:326–336. PMID: 10639358.12. Almeida Da Silva PE, Palomino JC. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemother. 2011; 66:1417–1430. PMID: 21558086.13. Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009; 13:1320–1330. PMID: 19861002.14. Morgan M, Kalantri S, Flores L, Pai M. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2005; 5:62. PMID: 16050959.
Article15. Hillemann D, Rüsch-Gerdes S, Richter E. Evaluation of the GenoType MTBDRplus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol. 2007; 45:2635–2640. PMID: 17537937.16. Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralized use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011; 377:1495–1505. PMID: 21507477.17. Chen J, Iannone MA, Li MS, Taylor JD, Rivers P, Nelsen AJ, et al. A microsphere-based assay for multiplexed single nucleotide polymorphism analysis using single base chain extension. Genome Res. 2000; 10:549–557. PMID: 10779497.
Article18. Armstrong B, Stewart M, Mazumder A. Suspension arrays for high throughput, multiplexed single nucleotide polymorphism genotyping. Cytometry. 2000; 40:102–108. PMID: 10805929.
Article19. Kellar KL, Iannone MA. Multiplexed microsphere-based flow cytometric assays. Exp Hematol. 2002; 30:1227–1237. PMID: 12423675.
Article20. Nolan JP, Mandy FF. Suspension array technology: new tools for gene and protein analysis. Cell Mol Biol (Noisy-le-grand). 2001; 47:1241–1256. PMID: 11838973.21. Kim SJ, Bai GH, Hong YP. Drug-resistant tuberculosis in Korea, 1994. Int J Tuberc Lung Dis. 1997; 1:302–308. PMID: 9432384.22. Dunbar SA. Application of Luminex xMAP technology for rapid, high-throughput multiplexed nucleic acid detection. Clin Chim Acta. 2006; 363:71–82. PMID: 16102740.23. Lee SH, Walker DR, Creqan PB, Boerma HR. Comparison of four flow cytometric SNP detection assays and their use in plant improvement. Theor Appl Genet. 2004; 110:167–174. PMID: 15551036.
Article24. Bergval I, Sengstake S, Brankova N, Levterova V, Abadía V, Tadumaze N, et al. Combined species identification, genotyping, and drug resistance detection of Mycobacterium tuberculosis cultures by MLPA on a bead-based array. PLoS One. 2012; 7:e43240. PMID: 22916230.25. Gomgnimbou MK, Hernández-Neuta I, Panaiotov S, Bachiyska E, Palomino JC, Martin A, et al. Tuberculosis-spoligo-rifampin-isoniazid typing: an all-in-one assay technique for surveillance and control of multidrug-resistant tuberculosis on Luminex devices. J Clin Microbiol. 2013; 51:3527–3534. PMID: 23966495.
Article26. Bortolin S, Black M, Modi H, Boszko I, Kobler D, Fieldhouse D, et al. Analytical validation of the tag-it high-throughput microsphere-based universal array genotyping platform: application to the multiplex detection of a panel of thrombophilia-associated single-nucleotide polymorphisms. Clin Chem. 2004; 50:2028–2036. PMID: 15364887.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Detection of First-Line Anti-Tuberculosis Drug Resistance Mutations by Allele-Specific Primer Extension on a Microsphere-Based Platform
- Evaluation of the QuantaMatrix Multiplexed Assay Platform for Molecular Diagnosis of Multidrug- and Extensively Drug-Resistant Tuberculosis Using Clinical Strains Isolated in Myanmar
- Rapid Detection of Rifampin Resistant Mycobacterium tuberculosis Using the Line Probe Assay
- Delamanid, Bedaquiline, and Linezolid Minimum Inhibitory Concentration Distributions and Resistance-related Gene Mutations in Multidrug-resistant and Extensively Drug-resistant Tuberculosis in Korea
- Development of Oligonucleotide Chip for Detection of Drug-Resistant Mycobacterium Tuberculosis