Intest Res.
2017 Jan;15(1):118-123. 10.5217/ir.2017.15.1.118.
Intestinal barrier integrity and function in infants with cholestasis
- Affiliations
-
- 1Department of Pediatrics, Faculty of Medicine, Assiut University, Assiut, Egypt. nhi-af@hotmail.com
- 2Department of Clinical Pathology, Faculty of Medicine, Assiut University, Assiut, Egypt.
- 3Department of Biochemistry, Faculty of Medicine, Minya University, Minya, Egypt.
- KMID: 2368494
- DOI: http://doi.org/10.5217/ir.2017.15.1.118
Abstract
- BACKGROUND/AIMS
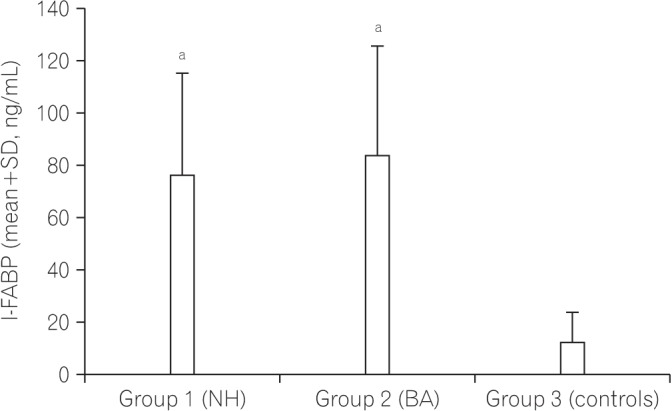
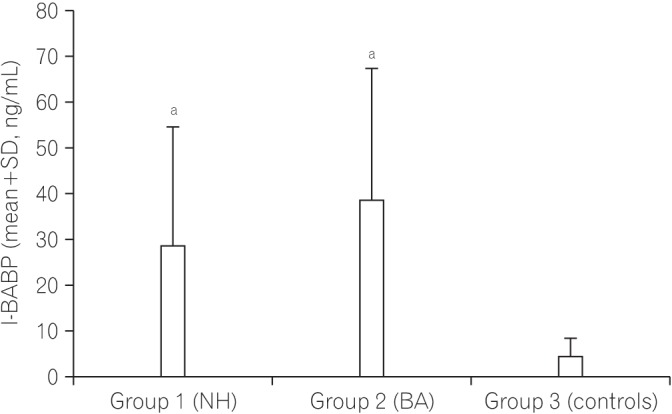
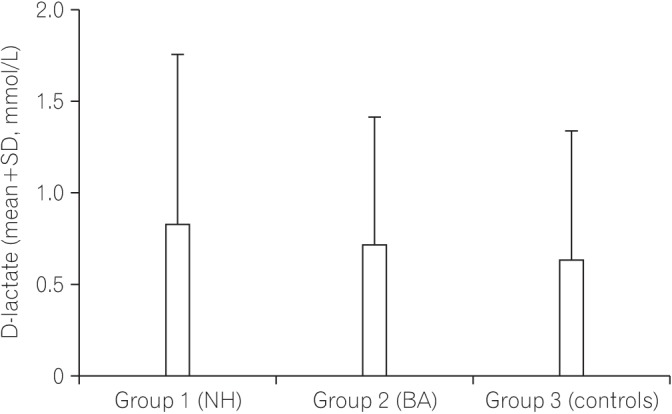
The safety of the human body is maintained by effective monitoring of the mucosal surface integrity and protection against potentially harmful compounds. This function of the gut called intestinal barrier function can be affected by cholestasis and the absence of bile in the intestinal lumen. We aimed to determine whether the gut barrier integrity is impaired in infants with cholestasis by evaluation of the intestinal fatty acid binding proteins (I-FABP) and ileal bile acid binding protein (I-BABP) as markers of intestinal epithelial cell damage and plasma D-lactate level as a marker of gut wall permeability.
METHODS
This case-control study included 53 infants with cholestasis and 29 controls. Serum levels of I-FABP, I-BABP, and D-lactate were measured in all subjects.
RESULTS
Both groups of patients with neonatal hepatitis and biliary atresia showed significantly higher levels of I-FABP and I-BABP than the controls. There were no differences in the serum D-lactate level between the cases and controls. There was no difference between the two groups of patients (I and II) regarding any of the parameters studied. No significant correlations between serum levels of I-FABP, I-BABP, or D-lactate and total or direct bilirubin levels were found in the cholestatic infants.
CONCLUSIONS
The intestinal epithelial barrier integrity is breached nearly in all parts of the intestine in infants with cholestasis. Further research is recommended to determine the impact of this finding on the management of these infants. The relationship between physical intestinal barrier damage and its functional failure remains subject for further research.
Keyword
MeSH Terms
Figure
Reference
-
1. Moyer V, Freese DK, Whitington PF, et al. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004; 39:115–128. PMID: 15269615.
Article2. Alaish SM, Smith AD, Timmons J, et al. Gut microbiota, tight junction protein expression, intestinal resistance, bacterial translocation and mortality following cholestasis depend on the genetic background of the host. Gut Microbes. 2013; 4:292–305. PMID: 23652772.
Article3. Assimakopoulos SF, Vagianos CE, Nikolopoulou VN. Intestinal barrier dysfunction in obstructive jaundice: current concepts in pathophysiology and potential therapies. Ann Gastroenterol. 2007; 20:116–123.4. Assimakopoulos SF, Vagianos CE, Patsoukis N, Georgiou C, Nikolopoulou V, Scopa CD. Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats. Acta Physiol Scand. 2004; 180:177–185. PMID: 14738476.
Article5. Brown WR, Kloppel TM. The liver and IgA: immunological, cell biological and clinical implications. Hepatology. 1989; 9:763–784. PMID: 2651270.
Article6. Wells CL, Jechorek RP, Erlandsen SL. Inhibitory effect of bile on bacterial invasion of enterocytes: possible mechanism for increased translocation associated with obstructive jaundice. Crit Care Med. 1995; 23:301–307. PMID: 7867356.7. Parks RW, Stuart Cameron CH, Gannon CD, Pope C, Diamond T, Rowlands BJ. Changes in gastrointestinal morphology associated with obstructive jaundice. J Pathol. 2000; 192:526–532. PMID: 11113871.
Article8. Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg. 2010; 2:61–69. PMID: 21160852.
Article9. Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009; 3:269–282. PMID: 19669378.
Article10. Welsh FK, Ramsden CW, MacLennan K, et al. Increased intestinal permeability and altered mucosal immunity in cholestatic jaundice. Ann Surg. 1998; 227:205–212. PMID: 9488518.
Article11. Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005; 2:416–422. PMID: 16265432.
Article12. Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009; 124:3–20. PMID: 19560575.
Article13. Thuijls G, Derikx JP, Grootjans J, Buurman WA, van Waardenburg DA. Intestinal barrier loss in sepsis. Neth J Crit Care. 2011; 15:199–203.14. Holmes JH 4th, Lieberman JM, Probert CB, et al. Elevated intestinal fatty acid binding protein and gastrointestinal complications following cardiopulmonary bypass: a preliminary analysis. J Surg Res. 2001; 100:192–196. PMID: 11592792.
Article15. Derikx JP, Evennett NJ, Degraeuwe PL, et al. Urine based detection of intestinal mucosal cell damage in neonates with suspected necrotising enterocolitis. Gut. 2007; 56:1473–1475. PMID: 17872576.
Article16. Hanssen SJ, Derikx JP, Vermeulen Windsant IC, et al. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann Surg. 2008; 248:117–125. PMID: 18580215.
Article17. de Haan JJ, Lubbers T, Derikx JP, et al. Rapid development of intestinal cell damage following severe trauma: a prospective observational cohort study. Crit Care. 2009; 13:R86. PMID: 19505335.
Article18. Derikx JP, Bijker EM, Vos GD, et al. Gut mucosal cell damage in meningococcal sepsis in children: relation with clinical outcome. Crit Care Med. 2010; 38:133–137. PMID: 19730255.
Article19. Pelsers MM, Namiot Z, Kisielewski W, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine: tissue distribution and clinical utility. Clin Biochem. 2003; 36:529–535. PMID: 14563446.
Article20. Derikx JP, Vreugdenhil AC, Van den Neucker AM, et al. A pilot study on the noninvasive evaluation of intestinal damage in celiac disease using I-FABP and L-FABP. J Clin Gastroenterol. 2009; 43:727–733. PMID: 19359998.
Article21. Toledo A, Yamaguchi J, Wang JY, Bass BL, Turner DJ, Strauch ED. Taurodeoxycholate stimulates intestinal cell proliferation and protects against apoptotic cell death through activation of NF-kappaB. Dig Dis Sci. 2004; 49:1664–1671. PMID: 15573924.
Article22. Yamaguchi J, Toledo A, Bass BL, et al. Taurodeoxycholate increases intestinal epithelial cell proliferation through c-myc expression. Surgery. 2004; 135:215–221. PMID: 14739857.
Article23. McConnell KW, Coopersmith CM. Epithelial cells. Crit Care Med. 2005; 33:S520–S522. PMID: 16340439.
Article24. Parks RW, Halliday MI, McCrory DC, et al. Host immune responses and intestinal permeability in patients with jaundice. Br J Surg. 2003; 90:239–245. PMID: 12555304.
Article25. Sun XQ, Fu XB, Zhang R, et al. Relationship between plasma D(-)-lactate and intestinal damage after severe injuries in rats. World J Gastroenterol. 2001; 7:555–558. PMID: 11819828.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vitamin D Improves Intestinal Barrier Function in Cirrhosis Rats by Upregulating Heme Oxygenase-1 Expression
- Intestinal Permeability Regulation by Tight Junction: Implication on Inflammatory Bowel Diseases
- Evaluation of Intestinal Epithelial Barrier Function in Inflammatory Bowel Diseases Using Murine Intestinal Organoids
- Cellular and Molecular Basis of Intestinal Barrier Dysfunction in the Irritable Bowel Syndrome
- Sepsis induces variation of intestinal barrier function in different phase through nuclear factor kappa B signaling