Ann Dermatol.
2014 Jun;26(3):332-337.
Notch Intracellular Domain Expression in Various Skin Fibroproliferative Diseases
- Affiliations
-
- 1Department of Dermatology, School of Medicine, The Catholic University of Korea, Seoul, Korea. johnkang@catholic.ac.kr
Abstract
- BACKGROUND
The effects of the Notch signaling pathway in fibroproliferative skin diseases have not been fully elucidated.
OBJECTIVE
The aim of this study was to investigate the expression of activated Notch signaling molecules in various skin fibroproliferative diseases.
METHODS
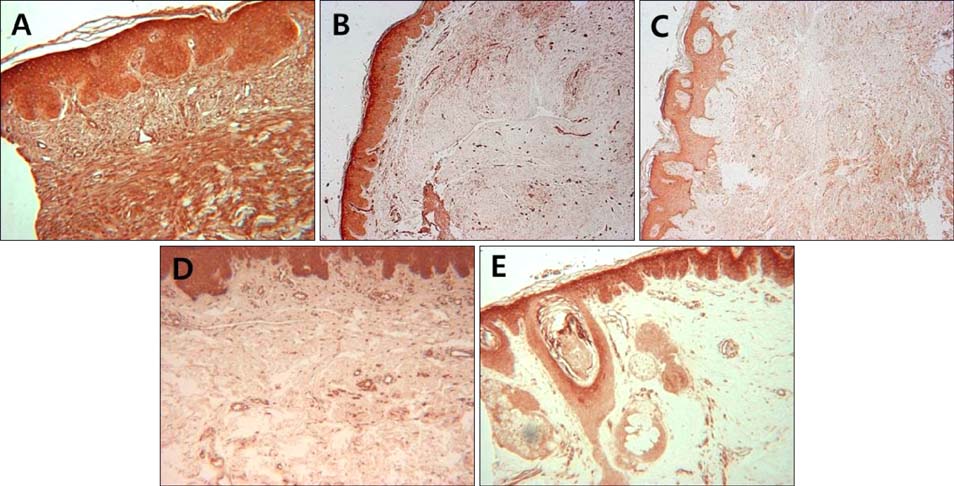
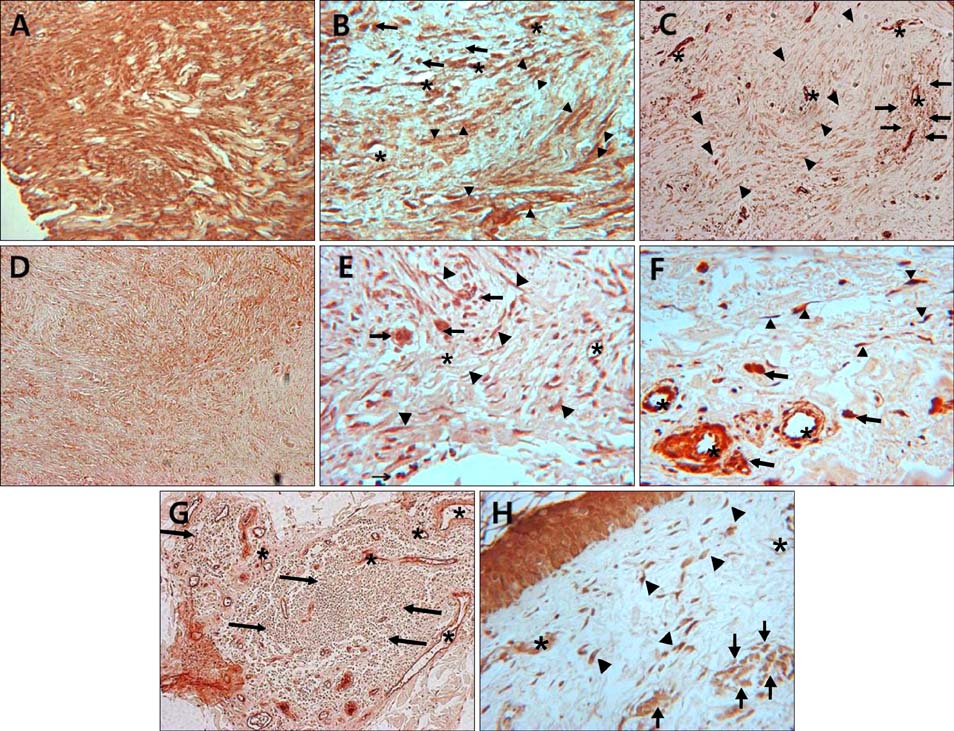
Immunohistochemical analysis of Notch intracellular domain (NICD) expression in keloid, hypertrophic scar, morphea, dermatofibroma, and normal control skin specimens was performed, and the clinical characteristics of patients with various skin fibroproliferative diseases were analyzed.
RESULTS
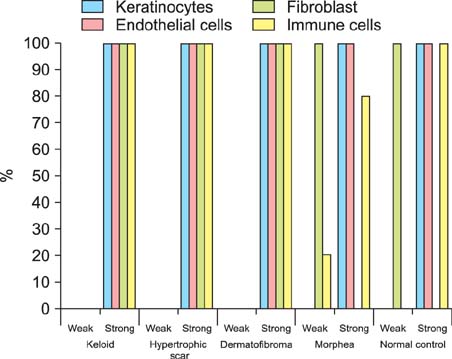
NICD was highly expressed in fibroblasts of keloids and moderately to highly expressed in hypertrophic scars and dermatofibromas, whereas low or no expression was detected in the fibroblasts of normal skin specimens and morpheas. NICD was constitutively expressed in keratinocytes, endothelial cells, and immune cells in normal skin specimens.
CONCLUSION
NICD was significantly expressed in human fibroproliferative skin disorders, especially keloids, suggesting that an activated Notch signaling pathway is involved in the pathogenesis of skin fibrosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009; 16:633–647.
Article2. Bolós V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007; 28:339–363.
Article3. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009; 137:216–233.
Article4. Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007; 134:1243–1251.
Article5. Kavian N, Servettaz A, Weill B, Batteux F. New insights into the mechanism of notch signalling in fibrosis. Open Rheumatol J. 2012; 6:96–102.
Article6. Aoyagi-Ikeda K, Maeno T, Matsui H, Ueno M, Hara K, Aoki Y, et al. Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor-{beta}-Smad3 pathway. Am J Respir Cell Mol Biol. 2011; 45:136–144.
Article7. Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002; 8:979–986.
Article8. Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. Br J Pharmacol. 2011; 163:141–172.
Article9. Dees C, Zerr P, Tomcik M, Beyer C, Horn A, Akhmetshina A, et al. Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum. 2011; 63:1396–1404.
Article10. Dees C, Tomcik M, Zerr P, Akhmetshina A, Horn A, Palumbo K, et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis. 2011; 70:1304–1310.
Article11. Shih B, Garside E, McGrouther DA, Bayat A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010; 18:139–153.
Article12. Zavadil J, Cermak L, Soto-Nieves N, Böttinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004; 23:1155–1165.
Article13. Beer TW, Lam MH, Heenan PJ. Tumors of fibrous tissue involving the skin. In : Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Lever's histopathology of the skin. 10th ed. Phyiladelphia: Lippincott Williams & Wilkins;2009. p. 969–980.14. Syed F, Bayat A. Notch signaling pathway in keloid disease: enhanced fibroblast activity in a Jagged-1 peptide-dependent manner in lesional vs. extralesional fibroblasts. Wound Repair Regen. 2012; 20:688–706.
Article15. Okuyama R, Tagami H, Aiba S. Notch signaling: its role in epidermal homeostasis and in the pathogenesis of skin diseases. J Dermatol Sci. 2008; 49:187–194.
Article16. Lin HY, Kao CH, Lin KM, Kaartinen V, Yang LT. Notch signaling regulates late-stage epidermal differentiation and maintains postnatal hair cycle homeostasis. PLoS One. 2011; 6:e15842.
Article17. Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, et al. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996; 86:531–542.
Article18. Matsuno Y, Coelho AL, Jarai G, Westwick J, Hogaboam CM. Notch signaling mediates TGF-β1-induced epithelial-mesenchymal transition through the induction of Snai1. Int J Biochem Cell Biol. 2012; 44:776–789.
Article19. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003; 112:1776–1784.
Article20. Nyhan KC, Faherty N, Murray G, Cooey LB, Godson C, Crean JK, et al. Jagged/Notch signalling is required for a subset of TGFβ1 responses in human kidney epithelial cells. Biochim Biophys Acta. 2010; 1803:1386–1395.
Article21. Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008; 105:6392–6397.
Article22. Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005; 16:667–675.
Article23. Svegliati S, Cancello R, Sambo P, Luchetti M, Paroncini P, Orlandini G, et al. Platelet-derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic sclerosis fibroblasts. J Biol Chem. 2005; 280:36474–36482.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Significance of NOTCH Pathway in the Development of Fibrosis in Systemic Sclerosis
- The cephalometric study on the depth of the mandibular antegonial notch as on indicator of mandibular growth pattern
- Vascular Endothelial Growth Factor Effect on Notch 1 Expression and Proliferation of Fibroblast
- A roentgenocephalometric study on the depth of the antegonial notch and the craniofacial morphology in Class III malocclusion
- Keloid Scarring: Understanding the Genetic Basis, Advances, and Prospects