Ann Dermatol.
2014 Jun;26(3):289-295.
Scalded Skin of Rat Treated by Using Fibrin Glue Combined with Allogeneic Bone Marrow Mesenchymal Stem Cells
- Affiliations
-
- 1Institute of Bioengineering, Zhejiang Academy of Medical Sciences, Hangzhou, China. zhangwy61@163.com
Abstract
- BACKGROUND
It is difficult to achieve satisfactory results with the traditional treatment of large-area skin defects and deep burns.
OBJECTIVE
To test the treatment effect of an active dressing film made of a mixture of fibrin glue and bone marrow mesenchymal stem cells (BMSCs) for repairing burn wounds on the skin of rats.
METHODS
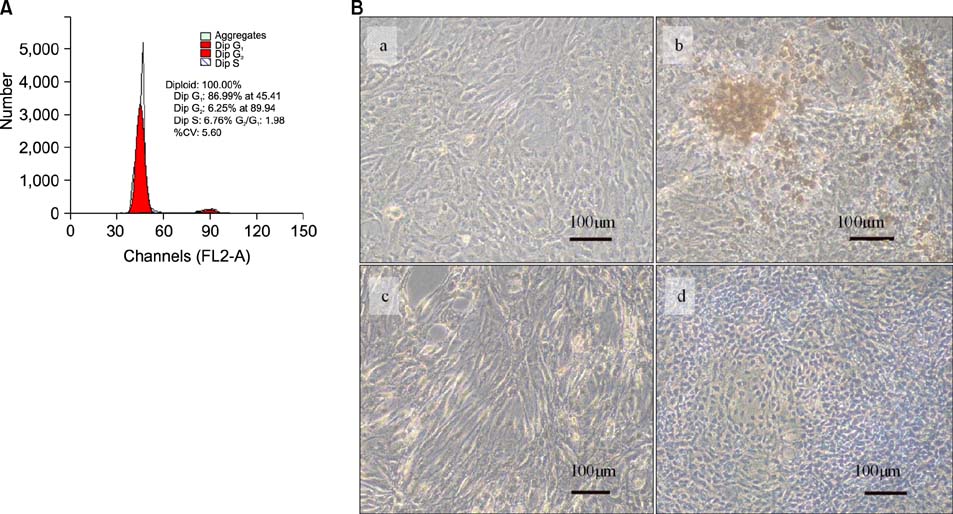
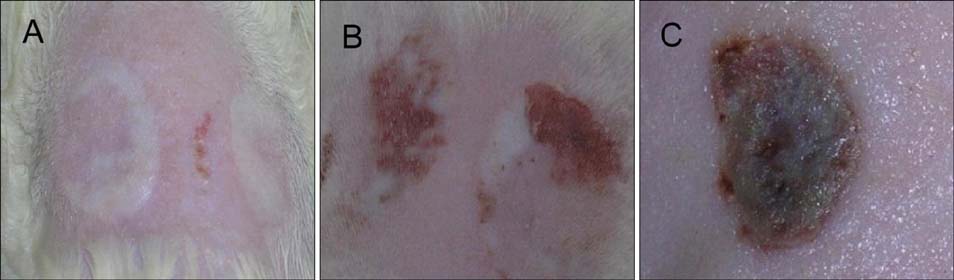
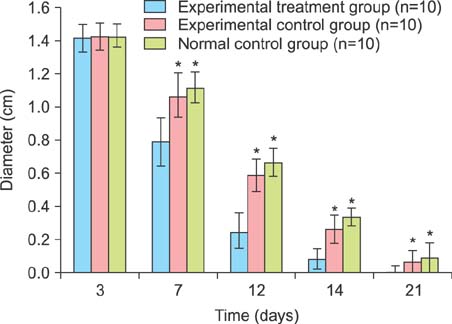
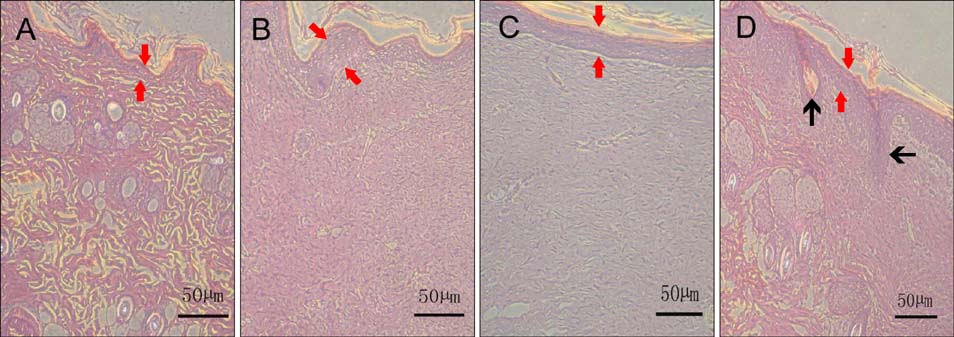
Two scald wounds were made on the back of each rat. A total of 30 scald wounds were randomly divided into 3 groups, with 10 wounds in each group. In the experimental treatment group, the scald wounds were covered with the fibrin glue and BMSC mixture. The wounds of the experimental control group were covered with fibrin glue only. No intervention was administered to the blank control group. Thirty days after treatment, pathological sections were cut from the scalded local tissues of all rats from the 3 groups and observed with a microscope.
RESULTS
The speed of scald wound healing in the experimental treatment group was faster than the other 2 groups. In the experimental treatment group, histopathological analysis revealed that the sebaceous glands showed obviously proliferous at the edge of the new tissue and gradually extended to the deep dermal layer of the new tissue.
CONCLUSION
BMSCs may have an active role in promoting skin tissue repair and generating skin appendages. Allogeneic BMSCs mixed with fibrin glue can contribute to the quick formation of a film-like gel over the scald wounds, which might be of significance for emergency treatment and skin-grafting operations.
Keyword
MeSH Terms
Figure
Reference
-
1. Sheridan R. Closure of the excised burn wound: autografts, semipermanent skin substitutes, and permanent skin substitutes. Clin Plast Surg. 2009; 36:643–651.
Article2. Xia ZF. Development and status quo of the fermanent skin substitutes. Med J Chin PLA. 2003; 28:389–391.3. Ren GH, Pei GX. Clinical application of tissue-engineered tissues. Chin J Tissue Eng Res. 2006; 10:152–156.4. Middelkoop E, van den Bogaerdt AJ, Lamme EN, Hoekstra MJ, Brandsma K, Ulrich MM. Porcine wound models for skin substitution and burn treatment. Biomaterials. 2004; 25:1559–1567.
Article5. Liu P, Deng Z, Han S, Liu T, Wen N, Lu W, et al. Tissue-engineered skin containing mesenchymal stem cells improves burn wounds. Artif Organs. 2008; 32:925–931.
Article6. Huang S, Lu G, Wu Y, Jirigala E, Xu Y, Ma K, et al. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J Dermatol Sci. 2012; 66:29–36.
Article7. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.
Article8. Zhu L, Li Q, Lin L, Wu H, Zhu S, Yu L. Effects of simulated microgravity on repairing articular cartilage defects by chondrocyte-modified fibrin glue bracket in rabbits. Chin J Bone Jt Inj. 2008; 23:741–744.9. Tokuishi K, Yamamoto S, Anami K, Moroga T, Miyawaki M, Chujo M, et al. Successful application of subcutaneous adipose tissue with fibrin glue in conservative treatment of tracheobronchial rupture. Ann Thorac Surg. 2012; 94:1726–1729.
Article10. Dhua AK, Ratan SK, Aggarwal SK. Chylothorax after primary repair of esophageal atresia with tracheo-esophageal fistula: successful management by biological fibrin glue. APSP J Case Rep. 2012; 3:16.11. Miri Bonjar MR, Maghsoudi H, Samnia R, Saleh P, Parsafar F. Efficacy of fibrin glue on seroma formation after breast surgery. Int J Breast Cancer. 2012; 2012:643132.
Article12. Feng S, Hua L, Jin S, Yan Z, Zhao Y, Zhu Q. Making the rat scald model. Acta Universitatis Medicinalis Secondae Shanghai. 1996; 15:195–197.13. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418:41–49.
Article14. Li H, Fu X, Wang J. Primary experimental studies on differentiation of marrow mesenchymal stem cells into skin appendage cells in vivo. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006; 20:675–678.15. Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006; 326:725–736.
Article16. Fu X, Sun T, Lei Y, Bao X, Zhang C, Cheng Z. Distribution of nerve and blood vessels in regenerative sweat glands induced from bone marrow mesenchymal stem cells. J Trauma Surg. 2009; 11:388–392.17. Yang ZM, Fan ZF, Xie HQ, Qin TW, Peng WZ. Vascularization and its relationship to osteogenesis in transplantation of tissue engineered bone to repair segment defect of long bone. Chin J Microsurg. 2002; 25:119–122.18. Fang LJ, Fu XB, Sun TZ, Li JF, Cheng B, Yang YH, et al. An experimental study on the differentiation of bone marrow mesenchymal stem cells into vascular endothelial cells. Zhonghua Shao Shang Za Zhi. 2003; 19:22–24.19. Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000; 106:571–578.
Article20. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004; 363:1439–1441.
Article21. Fibbe WE, Noort WA. Mesenchymal stem cells and hematopoietic stem cell transplantation. Ann N Y Acad Sci. 2003; 996:235–244.
Article22. Chao YH, Wu HP, Chan CK, Tsai C, Peng CT, Wu KH. Umbilical cord-derived mesenchymal stem cells for hematopoietic stem cell transplantation. J Biomed Biotechnol. 2012; 2012:759503.
Article23. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005; 3:1894–1904.
Article24. Collet JP, Shuman H, Ledger RE, Lee S, Weisel JW. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci U S A. 2005; 102:9133–9137.
Article25. Liu W, Jawerth LM, Sparks EA, Falvo MR, Hantgan RR, Superfine R, et al. Fibrin fibers have extraordinary extensibility and elasticity. Science. 2006; 313:634.
Article26. Cheng K, Rai P, Plagov A, Lan X, Kumar D, Salhan D, et al. Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Exp Mol Pathol. 2013; 94:466–473.
Article27. Yang YD, Zhang WY, Fang GJ, Wo EK, He ZJ, Hong Y, et al. Cell sources for cartilage defect repair with rabbit bone marrow mesenchymal stem cells PCR amplification for sex-determining gene sequence. J Clin Rehabil Tissue Eng Res. 2007; 11:7341–7344.28. Kim JW, Lee JH, Lyoo YS, Jung DI, Park HM. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet Dermatol. 2013; 24:242–249. e52–e53.
Article29. Abdel Aziz MT, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, Ahmed HH, et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem. 2007; 40:893–899.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vivo Cartilage Formation Using Human Bone Marrow-Derived Mesenchymal Stem Cells Mixed with Fibrin Glue
- Bone tissue engineering using PLLA/HA composite scaffold and bone marrow mesenchymal stem cell
- Two Cases of Generalized Vitiligo after Allogeneic Stem Cell Transplantation
- The effects of undifferentiated mesenchymal stem cells on sinus bone grafting in rabbit
- The effects of Bio-Oss(R) as a scaffolds during sinus bone graft using mesenchymal stem cells in rabbit