Ann Dermatol.
2017 Feb;29(1):102-105. 10.5021/ad.2017.29.1.102.
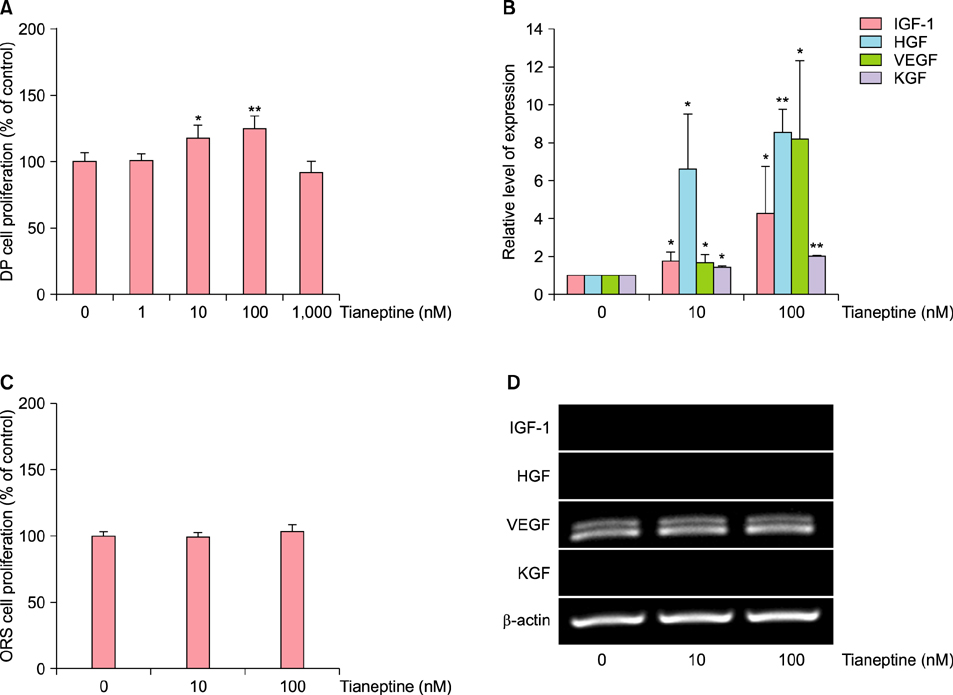
Attenuation of Dickkopf 1-Induced Hair Growth Inhibition in Cultured Human Hair Follicles by Tianeptine
- Affiliations
-
- 1Department of Immunology, Kyungpook National University School of Medicine, Daegu, Korea. ysung@knu.ac.kr
- KMID: 2368038
- DOI: http://doi.org/10.5021/ad.2017.29.1.102
Abstract
- No abstract available.
MeSH Terms
Figure
Reference
-
1. Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003; 8:46–55.
Article2. Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006; 119:391–393.
Article3. Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011; 124:1179–1182.
Article4. Kim HM, Lim YY, Kim MY, Son IP, Kim DH, Park SR, et al. Inhibitory effect of tianeptine on catagen induction in alopecia areata-like lesions induced by ultrasonic wave stress in mice. Clin Exp Dermatol. 2013; 38:758–767.
Article5. Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008; 128:262–269.
Article6. Kwack MH, Kim MK, Kim JC, Sung YK. Dickkopf 1 promotes regression of hair follicles. J Invest Dermatol. 2012; 132:1554–1560.
Article7. Philpott MP, Sanders DA, Kealey T. Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Invest Dermatol. 1994; 102:857–861.
Article8. Ahn SY, Pi LQ, Hwang ST, Lee WS. Effect of IGF-I on hair growth is related to the anti-apoptotic effect of IGF-I and up-regulation of PDGF-A and PDGF-B. Ann Dermatol. 2012; 24:26–31.
Article9. Shin SH, Bak SS, Kim MK, Sung YK, Kim JC. Baicalin, a flavonoid, affects the activity of human dermal papilla cells and promotes anagen induction in mice. Naunyn Schmiedebergs Arch Pharmacol. 2015; 388:583–586.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of TNF-alpha and Minoxidil on Human Hair Growth in Vitro
- Expression Pattern and Role of Klotho in Human Hair Follicles
- Effects of Substance P on the Hair Growth in Human Hair Follicle Organ Culture
- Growth Factors Influencing the Morphology and Growth Rate of Hair Follicles in a Human Hair Organ Culture
- The Effect of Calcitonin Gene-Related Peptide on Hair Growth in Vitro