Restor Dent Endod.
2017 Feb;42(1):39-47. 10.5395/rde.2017.42.1.39.
In vitro study of Streptococcus mutans adhesion on composite resin coated with three surface sealants
- Affiliations
-
- 1Department of Medical and Biological Engineering, Graduate School, Daegu, Korea.
- 2Department of Dental Biomaterials, School of Dentistry, Daegu, Korea. tykwon@knu.ac.kr
- 3Institute for Biomaterials Research and Development, Kyungpook National University, Daegu, Korea.
- KMID: 2367320
- DOI: http://doi.org/10.5395/rde.2017.42.1.39
Abstract
OBJECTIVES
Although the coating of surface sealants to dental composite resin may potentially reduce bacterial adhesion, there seems to be little information regarding this issue. This preliminary in vitro study investigated the adhesion of Streptococcus mutans (S. mutans) on the dental composite resins coated with three commercial surface sealants.
MATERIALS AND METHODS
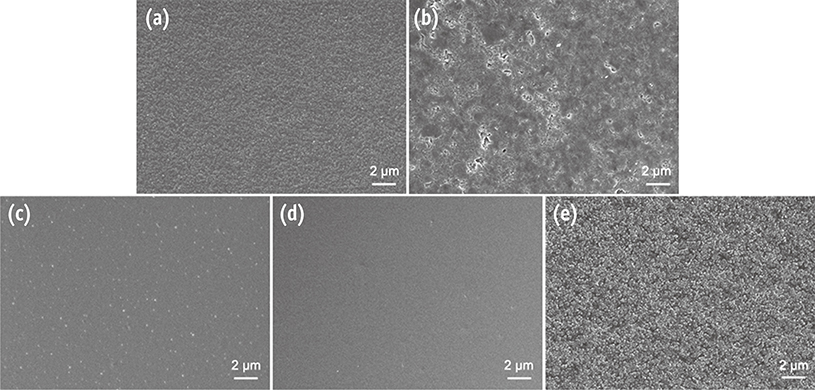
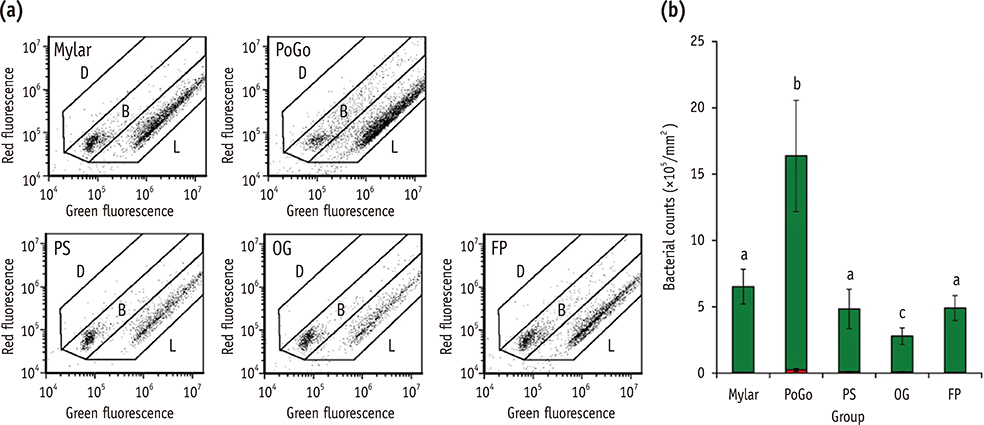
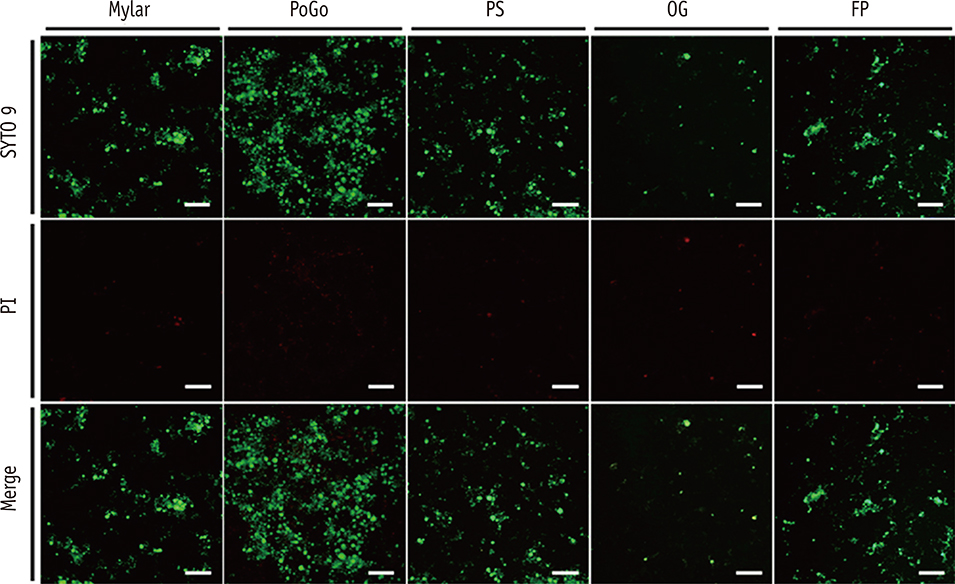
Composite resin (Filtek Z250) discs (8 mm in diameter, 1 mm in thickness) were fabricated in a mold covered with a Mylar strip (control). In group PoGo, the surfaces were polished with PoGo. In groups PS, OG, and FP, the surfaces polished with PoGo were coated with the corresponding surface sealants (PermaSeal, PS; OptiGuard, OG; Fortify Plus, FP). The surfaces of the materials and S. mutans cells were characterized by various methods. S. mutans adhesion to the surfaces was quantitatively evaluated using flow cytometry (n = 9).
RESULTS
Group OG achieved the lowest water contact angle among all groups tested (p < 0.001). The cell surface of S. mutans tested showed hydrophobic characteristics. Group PoGo exhibited the greatest bacterial adhesion among all groups tested (p < 0.001). The sealant-coated groups showed statistically similar (groups PS and FP, p > 0.05) or significantly lower (group OG, p < 0.001) bacterial adhesion when compared with the control group.
CONCLUSIONS
The application of the surface sealants significantly reduced S. mutans adhesion to the composite resin polished with the PoGo.
MeSH Terms
Figure
Reference
-
1. Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater. 1997; 13:258–269.
Article2. Cilli R, de Mattos MC, Honorio HM, Rios D, de Araujo PA, Prakki A. The role of surface sealants in the roughness of composites after a simulated toothbrushing test. J Dent. 2009; 37:970–977.
Article3. Bürgers R, Cariaga T, Müller R, Rosentritt M, Reischl U, Handel G, Hahnel S. Effects of aging on surface properties and adhesion of Streptococcus mutans on various fissure sealants. Clin Oral Investig. 2009; 13:419–426.
Article4. Dickinson GL, Leinfelder KF, Mazer RB, Russell CM. Effect of surface penetrating sealant on wear rate of posterior composite resins. J Am Dent Assoc. 1990; 121:251–255.
Article5. dos Santos PH, Consani S, Correr Sobrinho L, Coelho Sinhoreti MA. Effect of surface penetrating sealant on roughness of posterior composite resins. Am J Dent. 2003; 16:197–201.6. Prakki A, Ribeiro IW, Cilli R, Mondelli RF. Assessing the tooth-restoration interface wear resistance of two cementation techniques: effect of a surface sealant. Oper Dent. 2005; 30:739–746.7. Ramos RP, Chimello DT, Chinelatti MA, Dibb RG, Mondelli J. Effect of three surface sealants on marginal sealing of Class V composite resin restorations. Oper Dent. 2000; 25:448–453.8. dos Santos PH, Pavan S, Suzuki TY, Briso AL, Assunção WG, Sinhoreti MA, Correr-Sobrinho L, Consani S. Effect of fluid resins on the surface roughness and topography of resin composite restorations analyzed by atomic force microscope. J Mech Behav Biomed Mater. 2011; 4:433–439.
Article9. An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 1998; 43:338–348.
Article10. Türkün LS, Türkün M. The effect of one-step polishing system on the surface roughness of three esthetic resin composite materials. Oper Dent. 2004; 29:203–211.11. Martin MA, Pfaller MA, Massanari RM, Wenzel RP. Use of cellular hydrophobicity, slime production, and species identification markers for the clinical significance of coagulase-negative staphylococcal isolates. Am J Infect Control. 1989; 17:130–135.
Article12. Nostro A, Cannatelli MA, Crisafi G, Musolino AD, Procopio F, Alonzo V. Modifications of hydrophobicity, in vitro adherence and cellular aggregation of Streptococcus mutans by Helichrysum italicum extract. Lett Appl Microbiol. 2004; 38:423–427.
Article13. Zhou L, Tong Z, Wu G, Feng Z, Bai S, Dong Y, Ni L, Zhao Y. Parylene coating hinders Candida albicans adhesion to silicone elastomers and denture bases resin. Arch Oral Biol. 2010; 55:401–409.
Article14. Montanaro L, Campoccia D, Rizzi S, Donati ME, Breschi L, Prati C, Arciola CR. Evaluation of bacterial adhesion of Streptococcus mutans on dental restorative materials. Biomaterials. 2004; 25:4457–4463.
Article15. Kang SH, Lee HJ, Hong SH, Kim KH, Kwon TY. Influence of surface characteristics on the adhesion of Candida albicans to various denture lining materials. Acta Odontol Scand. 2013; 71:241–248.
Article16. Orth R, O'Brien-Simpson N, Dashper S, Walsh K, Reynolds E. An efficient method for enumerating oral spirochetes using flow cytometry. J Microbiol Methods. 2010; 80:123–128.
Article17. Pan H, Feng J, Cerniglia CE, Chen H. Effects of Orange II and Sudan III azo dyes and their metabolites on Staphylococcus aureus. J Ind Microbiol Biotechnol. 2011; 38:1729–1738.
Article18. Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 2009; 37:289–296.
Article19. Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995; 22:1–14.
Article20. Burdz TV, Wolfe J, Kabani A. Evaluation of sputum decontamination methods for Mycobacterium tuberculosis using viable colony counts and flow cytometry. Diagn Microbiol Infect Dis. 2003; 47:503–509.
Article21. Gunasekera TS, Attfield PV, Veal DA. A flow cytometry method for rapid detection and enumeration of total bacteria in milk. Appl Environ Microbiol. 2000; 66:1228–1232.
Article22. Filoche SK, Coleman MJ, Angker L, Sissons CH. A fluorescence assay to determine the viable biomass of microcosm dental plaque biofilms. J Microbiol Methods. 2007; 69:489–496.
Article23. Cai Y, Strømme M, Welch K. Bacteria viability assessment after photocatalytic treatment. 3 Biotech. 2014; 4:149–157.
Article24. Antonucci JM, Fowler BO, Dickens SH, Richards ND. Novel dental resins from trialkoxysilanes and dental monomers by in situ formation of oligomeric silyl ethers and silsesquioxanes. Polym Prepr. 2002; 43:633–634.25. Pereira SG, Osorio R, Toledano M, Nunes TG. Evaluation of two Bis-GMA analogues as potential monomer diluents to improve the mechanical properties of light-cured composite resins. Dent Mater. 2005; 21:823–830.
Article26. Tay FR, Pashley DH, Kapur RR, Carrilho MR, Hur YB, Garrett LV, Tay KC. Bonding BisGMA to dentin-a proof of concept for hydrophobic dentin bonding. J Dent Res. 2007; 86:1034–1039.
Article27. Wilson F, Heath JR, Watts DC. Finishing composite restorative materials. J Oral Rehabil. 1990; 17:79–87.
Article28. Ozel E, Korkmaz Y, Attar N, Karabulut E. Effect of one-step polishing systems on surface roughness of different flowable restorative materials. Dent Mater J. 2008; 27:755–764.
Article29. Korkmaz Y, Ozel E, Attar N, Aksoy G. The influence of one-step polishing systems on the surface roughness and microhardness of nanocomposites. Oper Dent. 2008; 33:44–50.
Article30. Kim MJ, Kim YK, Kim KH, Kwon TY. Shear bond strengths of various luting cements to zirconia ceramic: surface chemical aspects. J Dent. 2011; 39:795–803.
Article31. Hay KM, Dragila MI, Liburdy J. Theoretical model for the wetting of a rough surface. J Colloid Interface Sci. 2008; 325:472–477.
Article32. Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 1996; 7:201–211.
Article33. Claro-Pereira D, Sampaio-Maia B, Ferreira C, Rodrigues A, Melo LF, Vasconcelos MR. In situ evaluation of a new silorane-based composite resin's bioadhesion properties. Dent Mater. 2011; 27:1238–1245.
Article34. Kawai K, Leinfelder KF. Effect of surface-penetrating sealant on composite wear. Dent Mater. 1993; 9:108–113.
Article35. Busscher HJ, van Pelt AWJ, de Boer P, de Jong HP, Arends J. The effect of surface roughening of polymers on measured contact angles of liquids. Colloids Surf. 1984; 9:319–331.
Article36. Kim YK, Son JS, Kim KH, Kwon TY. Influence of surface energy parameters of dental self-adhesive resin cements on bond strength to dentin. J Adhes Sci Technol. 2013; 27:1778–1789.
Article37. Buergers R, Schneider-Brachert W, Hahnel S, Rosentritt M, Handel G. Streptococcal adhesion to novel low-shrink silorane-based restorative. Dent Mater. 2009; 25:269–275.
Article38. Rüttermann S, Bergmann N, Beikler T, Raab WH, Janda R. Bacterial viability on surface-modified resin-based dental restorative materials. Arch Oral Biol. 2012; 57:1512–1521.
Article39. Mei L, Busscher HJ, van der Mei HC, Chen Y, de Vries J, Ren Y. Oral bacterial adhesion forces to biomaterial surfaces constituting the bracket-adhesive-enamel junction in orthodontic treatment. Eur J Oral Sci. 2009; 117:419–426.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of surface sealant on surface roughness of dental composite with different surface roughness
- Effect of surface roughness of acrylic resin on the adhesion of bacteria
- Surface Roughness and Cariogenic Microbial Adhesion after Polishing of Smart Chromatic Technology-based Composite Resin
- Surface Roughness and Microbial Adhesion After Finishing of Alkasite Restorative Material
- Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin