Nat Prod Sci.
2016 Dec;22(4):238-245. 10.20307/nps.2016.22.4.238.
Simultaneous Determination of 11 Marker Compounds in Gumiganghwal-tang by HPLC-DAD and LC-MS
- Affiliations
-
- 1Department of Medical Biomaterials Engineering, College of Biomedical Science, Kangwon National University, Chuncheon 200-701, Korea. cjma@kangwon.ac.kr
- 2Institute of Bioscience and Biotechnology, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 2366690
- DOI: http://doi.org/10.20307/nps.2016.22.4.238
Abstract
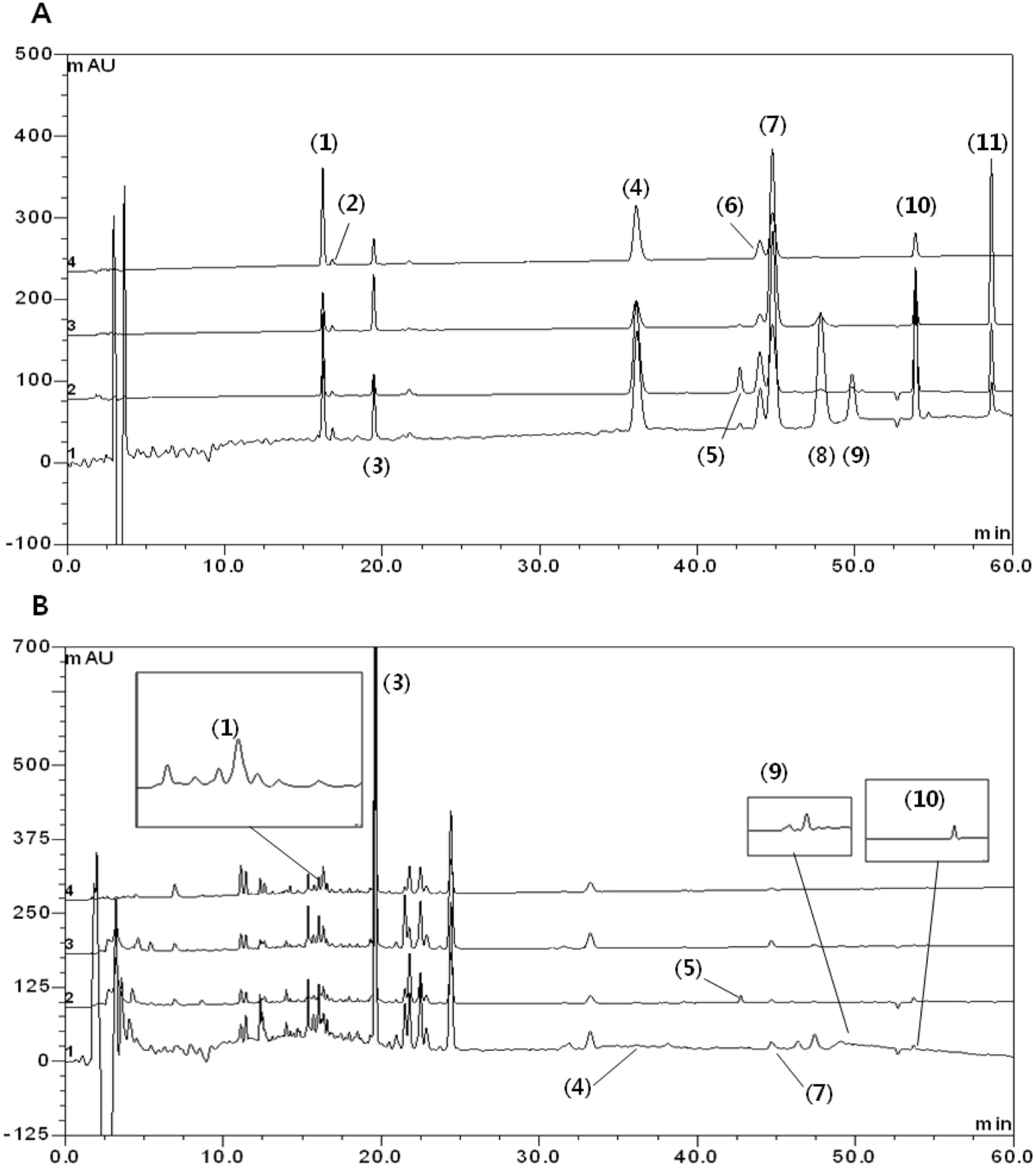
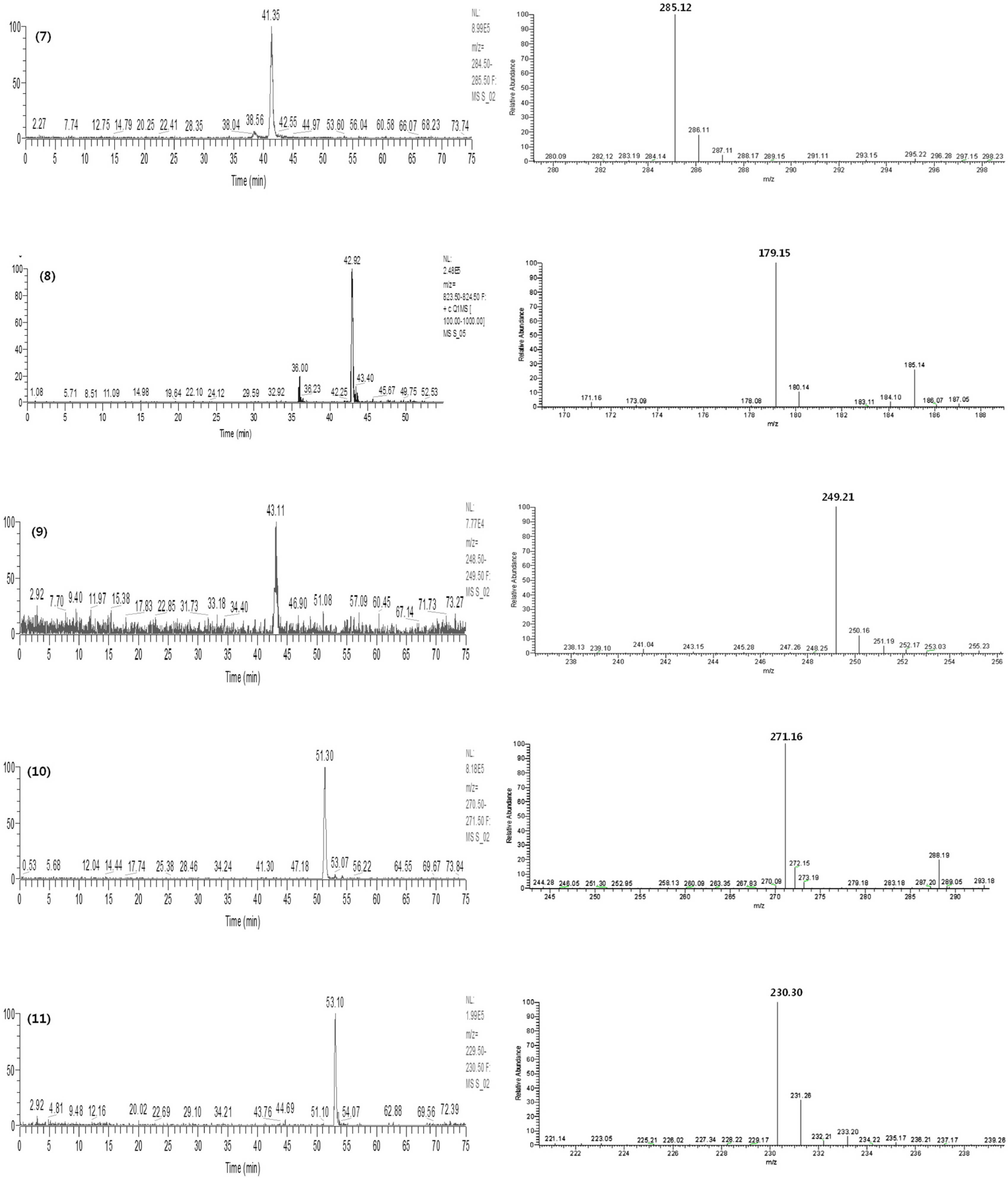
- Gumiganghwal-tang has been used for the treatment of common cold for a long-time. We developed an accurate and sensitive high performance liquid chromatography-diode array detection (HPLC-DAD) and electrospray ionization mass spectrometry method for the simultaneous determination of ferulic acid, baicalin, bergapten, methyl eugenol, glycyrrhizin, oxypeucedanin, wogonin, nodakenin, atractylenolide III, imperatorin, and atractylenolide I in Gumiganghwal-tang samples. The analytes were separated on a Shiseido C18 column (5 µm, 4.6 mm I.D. × 250 mm) with gradient elution with acetonitrile and 0.1% trifluoroacetic acid. Eleven compounds were quantitatively determined by HPLC-DAD and identified by LC-MS data. We also validated this method. The calibration curves of all the compounds showed good linear regression. The limits of detection and the limits of quantification ranged from 0.04 to 0.63 and from 0.12 to 1.92 µg/mL, respectively. The relative standard deviation values of intra- and inter-days of this method represented less than 2.9%. The recoveries were found to be in the range of 90.06 - 107.66%. The developed method has been successfully applied to the analysis of Gumiganghwal-tang samples. The established HPLC method could be used to quality control of Gumiganghwal-tang.
Keyword
MeSH Terms
Figure
Reference
-
References
(1). Jiang W. Y.Trends Pharmacol. Sci. 2005. 26:558–563.(2). Liang Y. Z.., Xie P. J.Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004. 812:53–70.(3). Bent S. J.Gen. Intern. Med. 2008. 23:854–859.(4). Firenzuoli F.., Gori L.Evid. Based Complement. Alternat. Med. 2007. 4:37–40.(5). Moon Y. H.., Go J. J.., Park J. Y.Kor. J. Pharmacogn. 1999. 30:18–24.(6). Kim S. J.., Jeong H. J.., Moon P. D.., Lee K. M.., Lee H. B.., Jung H. J.., Jung S. K.., Rhee H. K.., Yang D. C.., Hong S. H.., Kim H. M.Biol. Pharm. Bull. 2005. 28:233–237.(7). Lee M. Y.., Seo C. S.., Shin I. S.., Ha H.., Kim J. H.., Cho J. W.., Huh J. I.., Shin H. K.Regul. Toxicol. Pharmacol. 2012. 62:553–560.(8). Jung H. W.., Mahesh R.., Park J. H.., Boo Y. C.., Park K. M.., Park Y. K.Int. Immunopharmacol. 2010. 10:155–162.(9). Jung H. W.., Jung J. K.., Park Y. K.AsianPac. J. Allergy Immunol. 2011. 29:338–348.(10). Kang T. J.., Lee S. Y.., Singh R. P.., Agarwal R.., Yim D. S.Acta. Oncol. 2009. 48:895–900.(11). Okuyama E.., Hasegawa T.., Matsushita T.., Fujimoto H.., Ishibashi M.., Yamazaki M.Chem. Pharm. Bull. 2001. 49:154–160.(12). Chin Y. W.., Jung Y. H.., Chae H. S.., Yoon K. D.., Kim J.Bull. Korean Chem. Soc. 2011. 32:2132–2134.(13). Li S.., Han Q.., Qiao C.., Song J.., Lung Cheng C.., Xu H.Chin. Med. 2008. 3:DOI: 10.1186/1749-8546-3-7.(14). Choi D. W.., Kim J. H.., Cho S. Y.., Kim D. H.., Chang S. Y.Toxicology. 2002. 181–182:581–586.(15). Lin S. J.., Tseng H. H.., Wen K. C.., Suen T. T. J.Chromatogr. A. 1996. 730:17–23.(16). Liu Q.., Zhao J.., Yan L.., Yi J.., Song J.Zhongguo Zhong Yao Za Zhi. 2010. 35:708–710.(17). Zuo F.., Zhou Z. M.., Liu M. L.Biol. Pharm. Bull. 2001. 24:693–697.(18). Jiang P.., Shi R.., Wang Q.., Ma Y. M.., Cui H. Y.., Liu P.., Liu C. H.Biomed. Chromatogr. 2013. 27:874–881.(19). Samanidou V.., Tsagiannidis A.., Sarakatsianos I. J.Sep. Sci. 2012. 35:608–615.(20). Sun Q.., Chang L.., Ren Y.., Cao L.., Sun Y.., Du Y.., Shi X.., Wang Q.., Zhang L. J.Sep. Sci. 2012. 35:2897–2907.(21). Weon J. B.., Yang H. J.., Ma J. Y.., Ma C. J. J.Nat. Med. 2012. 66:510–515.(22). Shin I. S.., Seo C. S.., Lee M. Y.., Ha H. K.., Huh J. I.., Shin H. K. J.Ethnopharmacol. 2012. 141:350–356.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Simultaneous Determination of Five Compounds in Descurainia sophia by HPLC-DAD
- Simultaneous Determination of Eight Compounds in Lysimachia christinae by HPLC-DAD
- Simultaneous Determination of the Seven Phenylpropanoids in Xanthii Fructus Using a HPLC-PDA and LC-MS
- Simultaneous Quantification of Eight Compounds of Lonicera japonica by HPLC-DAD
- Quantitative Analysis of Flavonoid Glycosides in Sophora japonica and Sophora flavescens by HPLC-DAD