Korean J Ophthalmol.
2015 Oct;29(5):344-350. 10.3341/kjo.2015.29.5.344.
Comparison of Cytotoxic Effects on Rabbit Corneal Endothelium between Preservative-free and Preservative-containing Dorzolamide/timolol
- Affiliations
-
- 1Department of Ophthalmology, Korea University College of Medicine, Seoul, Korea. crisim@korea.ac.kr
- KMID: 2363809
- DOI: http://doi.org/10.3341/kjo.2015.29.5.344
Abstract
- PURPOSE
To evaluate and compare the toxic effects of eyedrops containing a fixed combination of 2.0% dorzolamide and 0.5% maleate timolol with or without preservatives on rabbit corneal endothelium.
METHODS
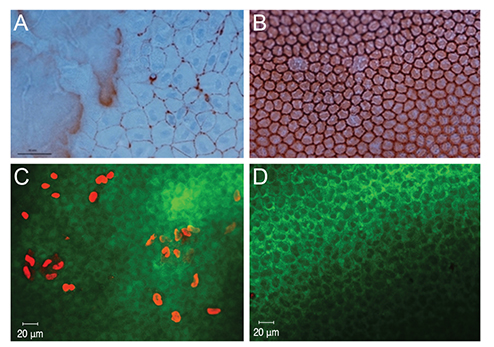
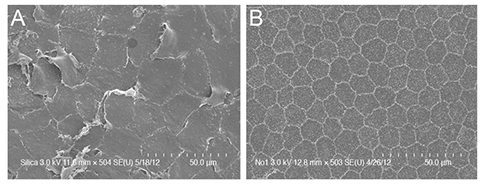
This study was performed with 22 eyes of New Zealand white rabbits. Dorzolamide/timolol eyedrops with preservative (Cosopt group) or without preservative (Cosopt-S group) were diluted with a balanced salt solution at a 1 : 1 ratio. We injected 0.1 mL of diluted Cosopt into the anterior chamber of left eyes and an equal volume of diluted Cosopt-S into the anterior chamber of right eyes. Corneal thickness, corneal haze, and conjunctival injection were measured before and 24 hours after treatment. Endothelial damage was compared between both eyes by vital staining (alizarin red/trypan blue staining), live/dead cell assay, TUNEL assay, and scanning electron microscopy.
RESULTS
Corneal endothelial damage was severe in the Cosopt group. Cosopt-treated eyes exhibited remarkable corneal edema and prominent apoptosis of endothelial cells. In addition, the live/dead cell assay revealed many dead cells in the endothelium, and scanning electron microscopy analysis showed that corneal endothelial cells exhibited a partial loss of microvilli on the surface as well as extensive destruction of intercellular junctions. However, in the Cosopt-S group, corneal edema was mild and the damage to the corneal endothelium was minimal.
CONCLUSIONS
The main cause of corneal endothelial toxicity was due to the preservative in the dorzolamide/timolol fixed combination eyedrops, and not the active ingredient. Thus, it appears to be safer to use preservative-free eyedrops during the early postoperative period.
Keyword
MeSH Terms
-
Animals
Anterior Chamber/drug effects
Apoptosis
Corneal Edema/chemically induced/*pathology
Disease Models, Animal
Drug Combinations
Endothelium, Corneal/drug effects/*pathology
In Situ Nick-End Labeling
Ophthalmic Solutions
Rabbits
Sulfonamides/administration & dosage/*toxicity
Thiophenes/administration & dosage/*toxicity
Timolol/administration & dosage/*toxicity
Drug Combinations
Ophthalmic Solutions
Sulfonamides
Thiophenes
Timolol
Figure
Reference
-
1. Lapalus P, Ettaiche M, Fredj-Reygrobellet D, et al. Cytotoxicity studies in ophthalmology. Lens Eye Toxic Res. 1990; 7:231–242.2. Guzman-Aranguez A, Calvo P, Ropero I, Pintor J. In vitro effects of preserved and unpreserved anti-allergic drugs on human corneal epithelial cells. J Ocul Pharmacol Ther. 2014; 30:790–798.3. Mantelli F, Tranchina L, Lambiase A, Bonini S. Ocular surface damage by ophthalmic compounds. Curr Opin Allergy Clin Immunol. 2011; 11:464–470.4. Baudouin C, Labbe A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010; 29:312–334.5. Ayaki M, Noda Y, Yaguchi S, et al. Cytotoxicity of antiglaucoma ophthalmic solutions for human corneal endothelial cells. Nihon Ganka Gakkai Zasshi. 2009; 113:576–582.6. Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001; 108:1943–1953.7. Inoue K, Iwasa M, Wakakura M, Tomita G. Effects of BAK-free travoprost treatment for 3 years in patients with normal tension glaucoma. Clin Ophthalmol. 2012; 6:1315–1319.8. Srinivas SP, Ong A, Zhai CB, Bonanno JA. Inhibition of carbonic anhydrase activity in cultured bovine corneal endothelial cells by dorzolamide. Invest Ophthalmol Vis Sci. 2002; 43:3273–3278.9. Wirtitsch MG, Findl O, Heinzl H, Drexler W. Effect of dorzolamide hydrochloride on central corneal thickness in humans with cornea guttata. Arch Ophthalmol. 2007; 125:1345–1350.10. Konowal A, Morrison JC, Brown SV, et al. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am J Ophthalmol. 1999; 127:403–406.11. Behrens A, Stark WJ, Pratzer KA, McDonnell PJ. Dynamics of small-incision clear cornea wounds after phacoemulsification surgery using optical coherence tomography in the early postoperative period. J Refract Surg. 2008; 24:46–49.12. Herretes S, Stark WJ, Pirouzmanesh A, et al. Inflow of ocular surface f luid into the anterior chamber after phacoemulsification through sutureless corneal cataract wounds. Am J Ophthalmol. 2005; 140:737–740.13. Taban M, Sarayba MA, Ignacio TS, et al. Ingress of India ink into the anterior chamber through sutureless clear corneal cataract wounds. Arch Ophthalmol. 2005; 123:643–648.14. Fantes FE, Hanna KD, Waring GO 3rd, et al. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990; 108:665–675.15. Ness PJ, Mamalis N, Werner L, et al. An anterior chamber toxicity study evaluating Besivance, AzaSite, and Ciprofloxacin. Am J Ophthalmol. 2010; 150:498–504.e1.16. Liu GS, Trope GE, Basu PK. Ultrastructural effects of topical betoptic, betagan, and timoptic on the rabbit corneal endothelium. J Ocul Pharmacol. 1989; 5:329–342.17. Ayaki M, Yaguchi S, Iwasawa A, Koide R. Cytotoxicity of ophthalmic solutions with and without preservatives to human corneal endothelial cells, epithelial cells and conjunctival epithelial cells. Clin Experiment Ophthalmol. 2008; 36:553–559.18. Egan CA, Hodge DO, McLaren JW, Bourne WM. Effect of dorzolamide on corneal endothelial function in normal human eyes. Invest Ophthalmol Vis Sci. 1998; 39:23–29.19. Giasson CJ, Nguyen TQ, Boisjoly HM, et al. Dorzolamide and corneal recovery from edema in patients with glaucoma or ocular hypertension. Am J Ophthalmol. 2000; 129:144–150.20. Baudouin C, de Lunardo C. Short-term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998; 82:39–42.21. Lewis RA, Katz GJ, Weiss MJ, et al. Travoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacy. J Glaucoma. 2007; 16:98–10.22. Konstas AG, Quaranta L, Katsanos A, et al. Twenty-four hour efficacy with preservative free tafluprost compared with latanoprost in patients with primary open angle glaucoma or ocular hypertension. Br J Ophthalmol. 2013; 97:1510–1515.23. Rouland JF, Traverso CE, Stalmans I, et al. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br J Ophthalmol. 2013; 97:196–200.24. Daull P, Buggage R, Lambert G, et al. A comparative study of a preservative-free latanoprost cationic emulsion (Catioprost) and a BAK-preserved latanoprost solution in animal models. J Ocul Pharmacol Ther. 2012; 28:515–523.25. Renieri G, Fuhrer K, Scheithe K, et al. Efficacy and tolerability of preservative-free eye drops containing a fixed combination of dorzolamide and timolol in glaucoma patients. J Ocul Pharmacol Ther. 2010; 26:597–603.26. Rasmussen CA, Kaufman PL, Kiland JA. Benzalkonium chloride and glaucoma. J Ocul Pharmacol Ther. 2014; 30:163–169.27. Maurice D, Perlman M. Permanent destruction of the corneal endothelium in rabbits. Invest Ophthalmol Vis Sci. 1977; 16:646–649.28. Valdez-Garcia JE, Lozano-Ramirez JF, Zavala J. Adult white New Zealand rabbit as suitable model for corneal endothelial engineering. BMC Res Notes. 2015; 8:28.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy and Safety of Preservative-containing and Preservative-free Brimonidine-Timolol Fixed Combination in Normal Tension Glaucoma
- Adherence to Preservative-Free Dorzolamide/Timolol Fixed Combination Assessed by Counting the Unused Single-Dose Units
- Effects of Corneal Toxicity and Conjunctival Injection of Preservative-free 0.1% Fluorometholone after Pediatric Strabismus Surgery
- Effect of the Preservative Benzalkonium Chloride in Prostaglandin Analogues on Corneal Sensitivity
- Effect of Dorzolamide on Corneal Endothelium