Int J Stem Cells.
2016 Nov;9(2):207-212. 10.15283/ijsc16049.
Autologous Bone Marrow Derived Stem Cells for the Treatment of Multiple Sclerosis
- Affiliations
-
- 1Department of Neurosurgery, The Lebanese-Canadian Hospital, Beirut, Lebanon. nassim@wp.eu
- 2Department of Hematology, The Lebanese-Canadian and Notre Dame University Hospital, Beirut, Lebanon.
- 3Department of Biology, University of Balamand, Balamand, Lebanon.
- 4Department of Neurology, The Lebanese-Canadian Hospital, Beirut, Lebanon.
- 5Neuro-rehabilitation, Physical Therapy, Beirut, Lebanon.
- KMID: 2362514
- DOI: http://doi.org/10.15283/ijsc16049
Abstract
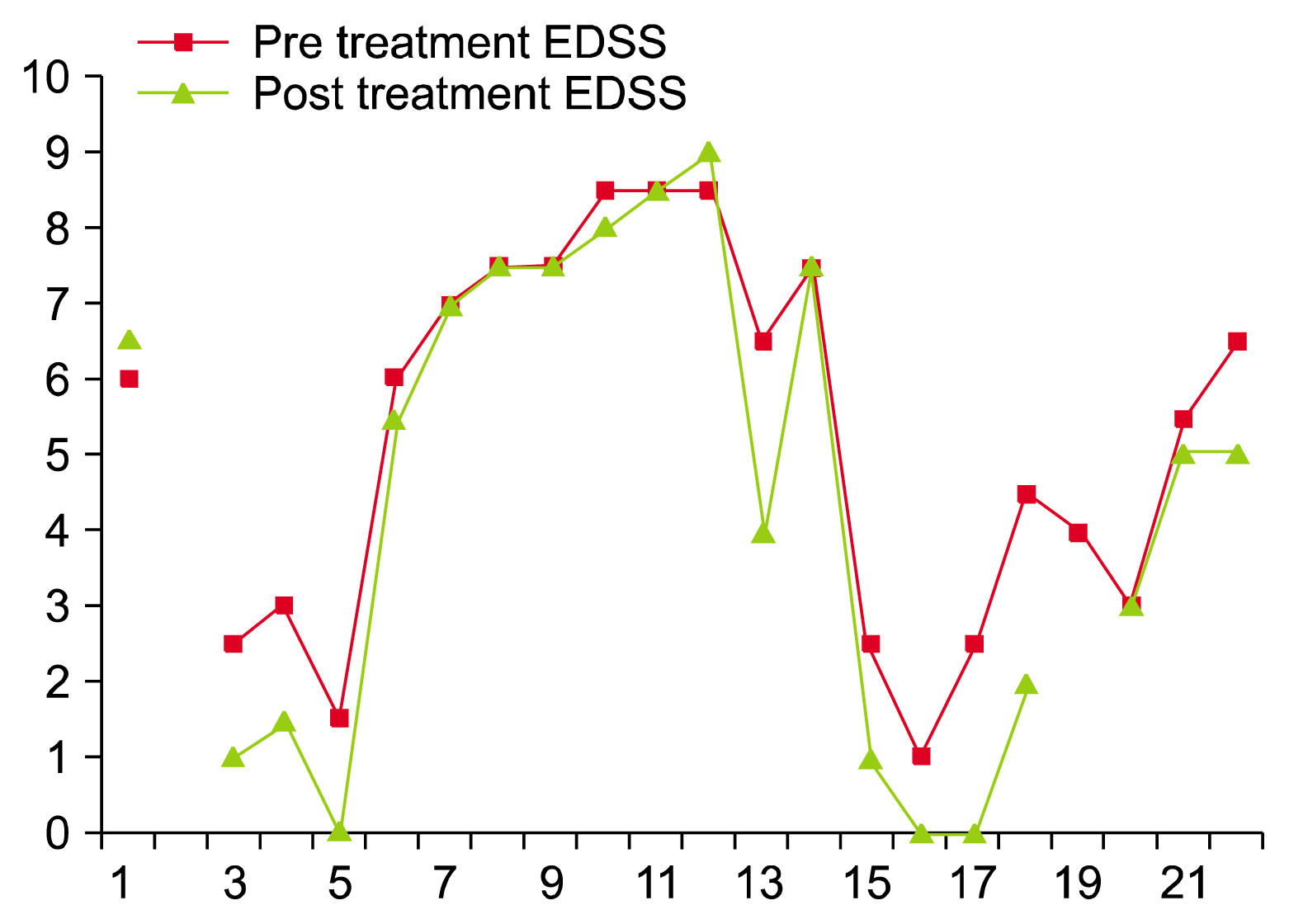
- Stem cell therapy, an evolving, progressive field of therapeutics has shown several successes in areas where classic treatments failed to prevent or stop disability. Starting in 2009, twenty two sequential patients with progressive Multiple Sclerosis (MS) courses were treated with Autologous Bone Marrow Mononuclear stem cells (BM-MNSCs). The cells were given both intravenously and intrathecally. Using the Expanded Disability Status Scale (EDSS) score for evaluation, our data indicates that the majority of the patients benefited on the average one point on the scale. This paper adds to the body of evidence suggesting the safety and efficacy of autologous BM-MNSCs in the treatment of MS and awaits validation through larger, randomized studies.
Keyword
Figure
Reference
-
References
1. Compston A, Coles A. Multiple sclerosis. Lancet. 2008; 372:1502–1517. DOI: 10.1016/S0140-6736(08)61620-7. PMID: 18970977.
Article2. Weinshenker BG. Epidemiology of multiple sclerosis. Neurol Clin. 1996; 142:291–308. DOI: 10.1016/S0733-8619(05)70257-7.
Article3. Barkhof F, Bruck W, De Groot CJ, Bergers E, Hulshof S, Geurts J, Polman CH, van der Valk P. Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch Neurol. 2003; 60:1073–1081. DOI: 10.1001/archneur.60.8.1073. PMID: 12925362.4. Navikas V, Link H. Review: cytokines and the pathogenesis of multiple sclerosis. J Neurosci Res. 1996; 45:322–333. DOI: 10.1002/(SICI)1097-4547(19960815)45:4<322::AID-JNR1>3.0.CO;2-B. PMID: 8872892.
Article5. Kingwell K. Disease mechanisms in MS: informing tactics to combat MS. Nature Reviews Neurology. 2012; 8:589. DOI: 10.1038/nrneurol.2012.218. PMID: 23128361.6. Chang A, Smith MC, Yin X, Fox RJ, Staugaitis SM, Trapp BD. Neurogenesis in the chronic lesions of multiple sclerosis. Brain. 2008; 131:2366–2375. DOI: 10.1093/brain/awn157. PMID: 18669500. PMCID: 2525445.
Article7. Calabresi PA. Diagnosis and management of multiple sclerosis. Am Fam Physician. 2004; 70:1935–1944. PMID: 15571060.8. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National multiple sclerosis society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology. 1996; 46:907–911. DOI: 10.1212/WNL.46.4.907. PMID: 8780061.
Article9. Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008; 9:839–855. DOI: 10.1038/nrn2480. PMID: 18931697.
Article10. Castro-Borrero W, Graves D, Frohman TC, Flores AB, Hardeman P, Logan D, Orchard M, Greenberg B, Frohman EM. Current and emerging therapies in multiple sclerosis: a systematic review. Ther Adv Neurol Disord. 2012; 5:205–220. DOI: 10.1177/1756285612450936. PMID: 22783370. PMCID: 3388530.
Article11. CAMMS223 Trial Investigators. Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, Norris K, Tandon PK. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008; 359:1786–1801. DOI: 10.1056/NEJMoa0802670. PMID: 18946064.
Article12. Bielekova B. Daclizumab therapy for multiple sclerosis. Neurotherapeutics. 2013; 10:55–67. DOI: 10.1007/s13311-012-0147-4. PMCID: 3557356.
Article13. Corboy JR, Miravalle AA. Emerging therapies for treatment of multiple sclerosis. J Inflamm Res. 2010; 3:53–59. DOI: 10.2147/JIR.S6558. PMID: 22096357. PMCID: 3218736.
Article14. Lunn JS, Sakowski SA, Federici T, Glass JD, Boulis NM, Feldman EL. Stem cell technology for the study and treatment of motor neuron diseases. Regen Med. 2011; 6:201–213. DOI: 10.2217/rme.11.6. PMID: 21391854. PMCID: 3154698.
Article15. Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res Ther. 2010; 1:37. DOI: 10.1186/scrt37. PMID: 21144012. PMCID: 3025439.
Article16. Abi Chahine NH, Wehbe TW, Hilal RA, Zoghbi VV, Melki AE, Habib EB. Treatment of cerebral palsy with stem cells: a report of 17 cases. Int J Stem Cells. 2016; 9:90–95. DOI: 10.15283/ijsc.2016.9.1.90. PMID: 27426090. PMCID: 4961108.
Article17. Trounson A. New perspectives in human stem cell therapeutic research. BMC Med. 2009; 7:29. DOI: 10.1186/1741-7015-7-29. PMID: 19519878. PMCID: 2702289.
Article18. Abi Chahine NH, Abou Saad ES, Melki SGE. How to maximize the success of stem cell autografts for neuroregeneration. J Stem Cell Res Ther. 2015; 5:271.
Article19. Sharma A, Gokulchandran N, Chopra G, Kulkarni P, Lohia M, Badhe P, Jacob VC. Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transplant. 2012; 21(Suppl 1):S79–S90. DOI: 10.3727/096368912X633798. PMID: 22507683.
Article20. Kuban JL, Hecht AB, Onderdonk T, O’Shea MN. Understanding the underlying beneficial biology of stem cells and the development and validation of more relevant animal models is required. Paneth Pediatr Res. 2010; 67:95–101.21. Payne N, Siatskas C, Barnard A, Bernard CC. The prospect of stem cells as multi-faceted purveyors of immune modulation, repair and regeneration in multiple sclerosis. Curr Stem Cell Res Ther. 2011; 6:50–62. DOI: 10.2174/157488811794480735.
Article22. Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci. 2003; 100:1364–1369. DOI: 10.1073/pnas.0336479100. PMID: 12538864. PMCID: 298778.
Article23. Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012; 11:150–156. DOI: 10.1016/S1474-4422(11)70305-2. PMID: 22236384. PMCID: 3279697.
Article24. Schäbitz WR, Krüger C, Pitzer C, Weber D, Laage R, Gassler N, Aronowski J, Mier W, Kirsch F, Dittgen T, Bach A, Sommer C, Schneider A. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GMCSF). J Cereb Blood Flow Metab. 2008; 28:29–43. DOI: 10.1038/sj.jcbfm.9600496.
Article25. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001; 105:369–377. DOI: 10.1016/S0092-8674(01)00328-2. PMID: 11348593.
Article26. Purandare C, Shitole DG, Belle V, Kedari A, Bora N, Joshi M. Therapeutic potential of autologous stem cell transplantation for cerebral palsy. Case Rep Transplant. 2012; 825289.
Article27. Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003; 361:47–49. DOI: 10.1016/S0140-6736(03)12111-3. PMID: 12517468.
Article28. Nakano-Doi A, Nakagomi T, Fujikawa M, Nakagomi N, Kubo S, Lu S, Yoshikawa H, Soma T, Taguchi A, Matsuyama T. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells. 2010; 28:1292–1302. PMID: 20517983.
Article29. Amin AH, Abd Elmageed ZY, Nair D, Partyka MI, Kadowitz PJ, Belmadani S, Matrougui K. Modified multi-potent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest. 2010; 90:985–996. DOI: 10.1038/labinvest.2010.86. PMID: 20440273. PMCID: 3154725.
Article30. Mimeault M, Hauke R, Batra SK. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007; 82:252–264. DOI: 10.1038/sj.clpt.6100301. PMID: 17671448.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- The Evolving Role of Myeloablative Chemotherapy with Stem Cell Transplantation for the Treatment of Autoimmune Disease
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro