Pediatr Infect Vaccine.
2016 Dec;23(3):202-208. 10.14776/piv.2016.23.3.202.
Occurrence Pattern of Intussusception according to the Introduction of Rotavirus Vaccine: An Observational Study at a University Hospital
- Affiliations
-
- 1Department of Pediatrics, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea. hkcho@gilhospital.com
- KMID: 2362479
- DOI: http://doi.org/10.14776/piv.2016.23.3.202
Abstract
- PURPOSE
Rotavirus vaccine (RV) was introduced in Korea since 2007, and intussusception (IS) remains an important safety concern. This study investigated the trend of IS occurrence related to RV as well as the temporal relevance between vaccination and IS in children.
METHODS
We collected data of the patient aged ≤18 years with IS admitted to Gachon University Gil Medical Center, 2003 to 2015. For the patients that have occurred since 2008, the immunization records of RV were collected. The proportion of cases <1 year was calculated by the year and the temporal relationship between vaccination and IS occurrence was analyzed.
RESULTS
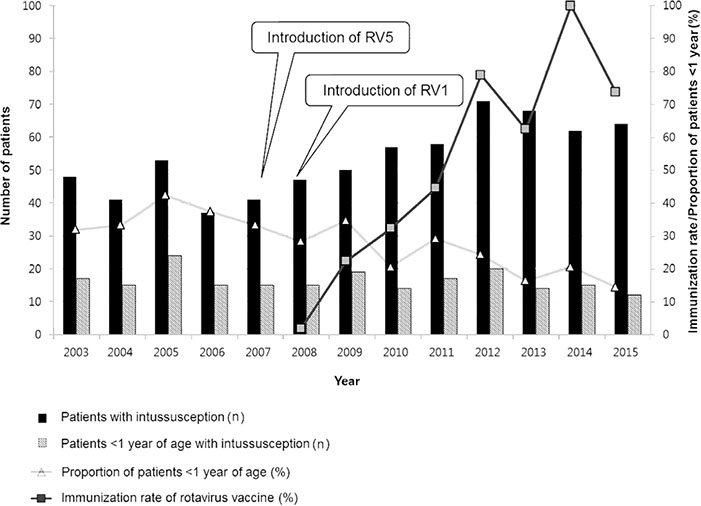
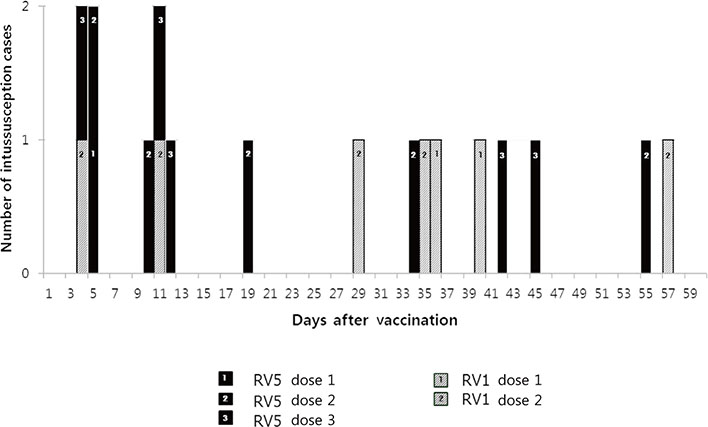
A total of 696 IS cases were noted. The cases <1 year were 30.7% (214/696). Although the incidence of all IS has increased over the 13-year period (from 74.1 in 2003 to 89.5 in 2015, linear by linear association, P=0.003), the incidence of IS <1 year has not increased (from 56.9 in 2003 to 53.3 in 2015, P=0.910), and the proportion of cases <1 year has decreased (from 35.4 in 2003 to 18.8 in 2015, P=0.000). Of 128 cases <1 year since 2008, 53.9% received RV. In the vaccinated group, 10 cases of IS occurred within 30 days, and eight cases did within 31 to 60 days. Numbers of IS after first, second, and third dose were three, 10, and five cases, respectively.
CONCLUSIONS
Occurrence of IS in children <1 year of age did not increase since the introduction of RV. Further monitoring is essential for evaluation of vaccine safety.
Keyword
MeSH Terms
Figure
Reference
-
1. Bryce J, Boschi-Pinto C, Shibuya K, Black RE;. WHO estimates of the causes of death in children. Lancet. 2005; 365:1147–1152.
Article2. Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006; 12:304–306.
Article3. Dennehy PH. Rotavirus vaccines: an update. Vaccine. 2007; 25:3137–3141.
Article4. Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006; 354:11–22.
Article5. Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006; 354:23–33.
Article6. Cortese MM, Parashar UD. Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009; 58(RR-2):1–25.7. Than VT, Jeong S, Kim W. Characterization of RotaTeq(R) vaccine-derived rotaviruses in South Korean infants with rotavirus gastroenteritis. J Med Virol. 2015; 87:112–116.
Article8. Park DK, Chung JY. The changes in the outbreak of rotavirus gastroenteritis in children after introduction of rotavirus vaccines: a retrospective study at a tertiary hospital. Korean J Pediatr Infect Dis. 2014; 21:167–173.
Article9. Sohn TY, Lee CJ, Kim YJ, Kang MJ, Kim SH, Lee SY, et al. Clinical and epidemiological study of 1,165 hospitalized cases of rotaviral gastroenteritis before and after the introduction of rotavirus vaccine, 2006-2013. Korean J Pediatr Infect Dis. 2014; 21:174–180.
Article10. Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis. 2010; 201:1617–1624.
Article11. Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's National Childhood vaccine schedule. Pediatr Infect Dis J. 2011; 30:1 Suppl. S25–S29.
Article12. Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011; 364:2283–2292.13. Velazquez FR, Colindres RE, Grajales C, Hernandez MT, Mercadillo MG, Torres FJ, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J. 2012; 31:736–744.
Article14. Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014; 370:503–512.
Article15. Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011; 29:3061–3066.
Article16. Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001; 344:564–572.
Article17. Belongia EA, Irving SA, Shui IM, Kulldorff M, Lewis E, Yin R, et al. Real-time surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine. Pediatr Infect Dis J. 2010; 29:1–5.
Article18. Haber P, Patel M, Izurieta HS, Baggs J, Gargiullo P, Weintraub E, et al. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States, February 1, 2006, to September 25, 2007. Pediatrics. 2008; 121:1206–1212.
Article19. Glass RI, Parashar UD. Rotavirus vaccines: balancing intussusception risks and health benefits. N Engl J Med. 2014; 370:568–570.
Article20. Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J. 2011; 30:1 Suppl. S1–S5.
Article21. Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia's National Immunization Program. Clin Infect Dis. 2013; 57:1427–1434.
Article22. Bauchau V, Van Holle L, Mahaux O, Holl K, Sugiyama K, Buyse H. Post-marketing monitoring of intussusception after rotavirus vaccination in Japan. Pharmacoepidemiol Drug Saf. 2015; 24:765–770.
Article23. Lee YY, Lee EB, Choi KH. Difference in the distribution of onset age of intussusception after rotavirus vaccination and according to the type of rotavirus vaccine: single medical center study. Yeungnam Univ J Med. 2015; 32:80–84.
Article24. Chungnam National University and Korea Centers for Disease Control and Prevention (KCDC). Korea National Immunization Survey. Seoul: Chungnam National University and KCDC;2012.25. Yang HI, Park EY, Kim MY. Korea Centers for Disease Control and Prevention (KCDC). National immunization survey in South Korea, 2013. Public Health Wkly Rep. 2013; 7:449–454.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rotavirus Vaccine
- Difference in the distribution of onset age of intussusception after rotavirus vaccination and according to the type of rotavirus vaccine: single medical center study
- Relationship between Pentavalent Rotavirus Vaccine and Intussusception: A Retrospective Study at a Single Center in Korea
- Changes in the Occurrence of Rotavirus Gastroenteritis before and after the Introduction of Rotavirus Vaccine among Hospitalized Pediatric Patients and Estimates of Rotavirus Vaccine Effectiveness
- The Changes in the Outbreak of Rotavirus Gastroenteritis in Children after Introduction of Rotavirus Vaccines: A Retrospective Study at a Tertiary Hospital