Yonsei Med J.
2017 May;58(3):631-636. 10.3349/ymj.2017.58.3.631.
Relationship between Pentavalent Rotavirus Vaccine and Intussusception: A Retrospective Study at a Single Center in Korea
- Affiliations
-
- 1Department of Pediatrics, Yonsei University College of Medicine, Severance Children's Hospital, Seoul, Korea. dskim6634@yuhs.ac
- KMID: 2419123
- DOI: http://doi.org/10.3349/ymj.2017.58.3.631
Abstract
- PURPOSE
Despite withdrawal of RotaShield® and the development of second generation live attenuated rotavirus vaccines, concerns remain regarding the relationship between rotavirus vaccine and intussusception. Nevertheless, since there is no study in Korea, we reviewed data from cases at Severance Children's Hospital to determine the association between rotavirus vaccine and intussusception.
MATERIALS AND METHODS
Patients coded as intussusception and following a prescription of RotaTeq® from 2007 to 2013 were reviewed. We calculated comparative incidence figures (CIFs) and 95% confidence intervals (CIs) to compare the risk of intussusception in Korea with the risk in the United States. Expected cases within the four-week post-vaccination window were calculated by applying rates of intussusception from data compiled by the Health Insurance Review and Assessment Service (for a five-year period) to numbers of vaccinations.
RESULTS
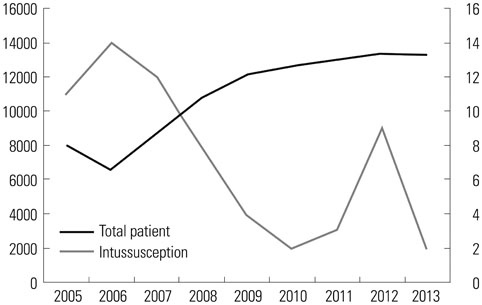
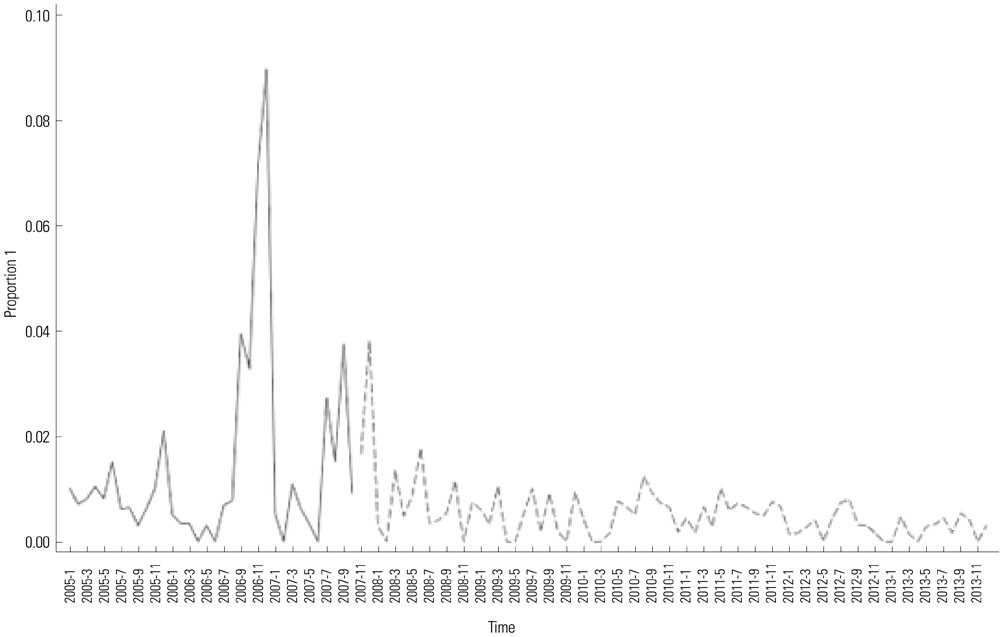
In total, 10530 doses of pentavalent rotavirus vaccine were administered. A total of 65 intussusception cases were diagnosed, although only two cases occurred within four weeks after vaccination. This was compared to six cases within 999123 doses in United States from April 2008 to March 2013 (CIF, 31.63; CI, 31.33-31.93). When we adjusted incidence rate differences for both countries, the CIF decreased to 7.05 (CI, 6.72-7.40). When we compared our identified cases with the expected cases from our hospital, there was no increased intussusception occurring within four weeks of vaccination.
CONCLUSION
We found no association between pentavalent rotavirus vaccine and intussusception. Therefore, rotavirus vaccination should be considered due to its benefits of preventing rotavirus-associated diseases.
MeSH Terms
-
Child, Preschool
Female
Hospitals
Humans
Incidence
Infant
Intussusception/epidemiology/*etiology
Male
Medical Records
Republic of Korea/epidemiology
Retrospective Studies
Risk
Rotavirus Vaccines/administration & dosage/*adverse effects
Time Factors
United States/epidemiology
Vaccination/statistics & numerical data
Vaccines, Attenuated/administration & dosage/*adverse effects
Rotavirus Vaccines
Vaccines, Attenuated
Figure
Reference
-
1. Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012; 12:136–141.
Article2. Kang HY, Kim KH, Kim JH, Kim HM, Kim J, Kim MS, et al. Economic evaluation of the national immunization program of rotavirus vaccination for children in Korea. Asia Pac J Public Health. 2013; 25:145–158.
Article3. Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001; 344:564–572.
Article4. Murphy TV, Smith PJ, Gargiullo PM, Schwartz B. The first rotavirus vaccine and intussusception: epidemiological studies and policy decisions. J Infect Dis. 2003; 187:1309–1313.
Article5. Kramarz P, France EK, Destefano F, Black SB, Shinefield H, Ward JI, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J. 2001; 20:410–416.
Article6. Centers for Disease Control and Prevention (CDC). Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999; 48:1007.7. Parashar UD, Alexander JP, Glass RI. Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006; 55:1–13.8. Cortese MM, Parashar UD. Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009; 58:1–25.9. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006; 354:11–22.
Article10. Velázquez FR, Colindres RE, Grajales C, Hernández MT, Mercadillo MG, Torres FJ, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J. 2012; 31:736–744.
Article11. Rotavirus vaccines: an update. Wkly Epidemiol Rec. 2009; 84:533–540.12. Haber P, Patel M, Pan Y, Baggs J, Haber M, Museru O, et al. Intussusception after rotavirus vaccines reported to US VAERS, 2006-2012. Pediatrics. 2013; 131:1042–1049.
Article13. Shui IM, Baggs J, Patel M, Parashar UD, Rett M, Belongia EA, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA. 2012; 307:598–604.
Article14. Zickafoose JS, Benneyworth BD, Riebschleger MP, Espinosa CM, Davis MM. Hospitalizations for intussusception before and after the reintroduction of rotavirus vaccine in the United States. Arch Pediatr Adolesc Med. 2012; 166:350–355.
Article15. Yen C, Tate JE, Steiner CA, Cortese MM, Patel MM, Parashar UD. Trends in intussusception hospitalizations among US infants before and after implementation of the rotavirus vaccination program, 2000-2009. J Infect Dis. 2012; 206:41–48.
Article16. Patel MM, López-Collada VR, Bulhões MM, De Oliveira LH, Bautista Márquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011; 364:2283–2292.
Article17. Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011; 29:3061–3066.
Article18. Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clin Infect Dis. 2013; 57:1427–1434.
Article19. Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014; 370:513–519.
Article20. Kim JS, Bae CW, Lee KY, Park MS, Choi YY, Kim KN, et al. Immunogenicity, reactogenicity and safety of a human rotavirus vaccine (RIX4414) in Korean infants: a randomized, double-blind, placebo-controlled, phase IV study. Hum Vaccin Immunother. 2012; 8:806–812.
Article21. Tate JE, Simonsen L, Viboud C, Steiner C, Patel MM, Curns AT, et al. Trends in intussusception hospitalizations among US infants, 1993-2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics. 2008; 121:e1125–e1132.
Article22. Jo DS, Nyambat B, Kim JS, Jang YT, Ng TL, Bock HL, et al. Population-based incidence and burden of childhood intussusception in Jeonbuk Province, South Korea. Int J Infect Dis. 2009; 13:e383–e388.
Article23. Konno T, Suzuki H, Kutsuzawa T, Imai A, Katsushima N, Sakamoto M, et al. Human rotavirus infection in infants and young children with intussusception. J Med Virol. 1978; 2:265–269.
Article24. Nicolas JC, Ingrand D, Fortier B, Bricout F. A one-year virological survey of acute intussusception in childhood. J Med Virol. 1982; 9:267–271.25. Mulcahy DL, Kamath KR, de Silva LM, Hodges S, Carter IW, Cloonan MJ. A two-part study of the aetiological role of rotavirus in intussusception. J Med Virol. 1982; 9:51–55.
Article26. Rennels MB, Parashar UD, Holman RC, Le CT, Chang HG, Glass RI. Lack of an apparent association between intussusception and wild or vaccine rotavirus infection. Pediatr Infect Dis J. 1998; 17:924–925.
Article27. Nakagomi T. Rotavirus infection and intussusception: a view from retrospect. Microbiol Immunol. 2000; 44:619–628.
Article28. Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014; 370:503–512.
Article29. Noel G, Minodier P, Merrot T. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014; 370:1766.
Article30. Justice F, Carlin J, Bines J. Changing epidemiology of intussusception in Australia. J Paediatr Child Health. 2005; 41:475–478.
Article31. Phua KB, Lee BW, Quak SH, Jacobsen A, Teo H, Vadivelu-Pechai K, et al. Incidence of intussusception in Singaporean children aged less than 2 years: a hospital-based prospective study. BMC Pediatr. 2013; 13:161.32. Khumjui C, Doung-ngern P, Sermgew T, Smitsuwan P, Jiraphongsa C. Incidence of intussusception among children 0-5 years of age in Thailand, 2001-2006. Vaccine. 2009; 27:Suppl 5. F116–F119.
Article33. Buettcher M, Baer G, Bonhoeffer J, Schaad UB, Heininger U. Three-year surveillance of intussusception in children in Switzerland. Pediatrics. 2007; 120:473–480.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rotavirus Vaccine
- Rotavirus Vaccines
- Difference in the distribution of onset age of intussusception after rotavirus vaccination and according to the type of rotavirus vaccine: single medical center study
- Occurrence Pattern of Intussusception according to the Introduction of Rotavirus Vaccine: An Observational Study at a University Hospital
- The laboratory test procedure to confirm rotavirus vaccine infection in severe complex immunodeficiency patients