J Pathol Transl Med.
2016 Nov;50(6):442-450. 10.4132/jptm.2016.07.08.
Detection of Human Papillomavirus in Korean Breast Cancer Patients by Real-Time Polymerase Chain Reaction and Meta-Analysis of Human Papillomavirus and Breast Cancer
- Affiliations
-
- 1Department of Pathology, Korea University Guro Hospital, Seoul, Korea. Ark@korea.ac.kr
- KMID: 2361992
- DOI: http://doi.org/10.4132/jptm.2016.07.08
Abstract
- BACKGROUND
Human papillomavirus (HPV) is a well-established oncogenic virus of cervical, anogenital, and oropharyngeal cancer. Various subtypes of HPV have been detected in 0% to 60% of breast cancers. The roles of HPV in the carcinogenesis of breast cancer remain controversial. This study was performed to determine the prevalence of HPV-positive breast cancer in Korean patients and to evaluate the possibility of carcinogenic effect of HPV on breast.
METHODS
Meta-analysis was performed in 22 case-control studies for HPV infection in breast cancer. A total of 123 breast cancers, nine intraductal papillomas and 13 nipple tissues of patients with proven cervical HPV infection were tested by real-time polymerase chain reaction to detect 28 subtypes of HPV. Breast cancers were composed of 106 formalin-fixed and paraffin embedded (FFPE) breast cancer samples and 17 touch imprint cytology samples of breast cancers.
RESULTS
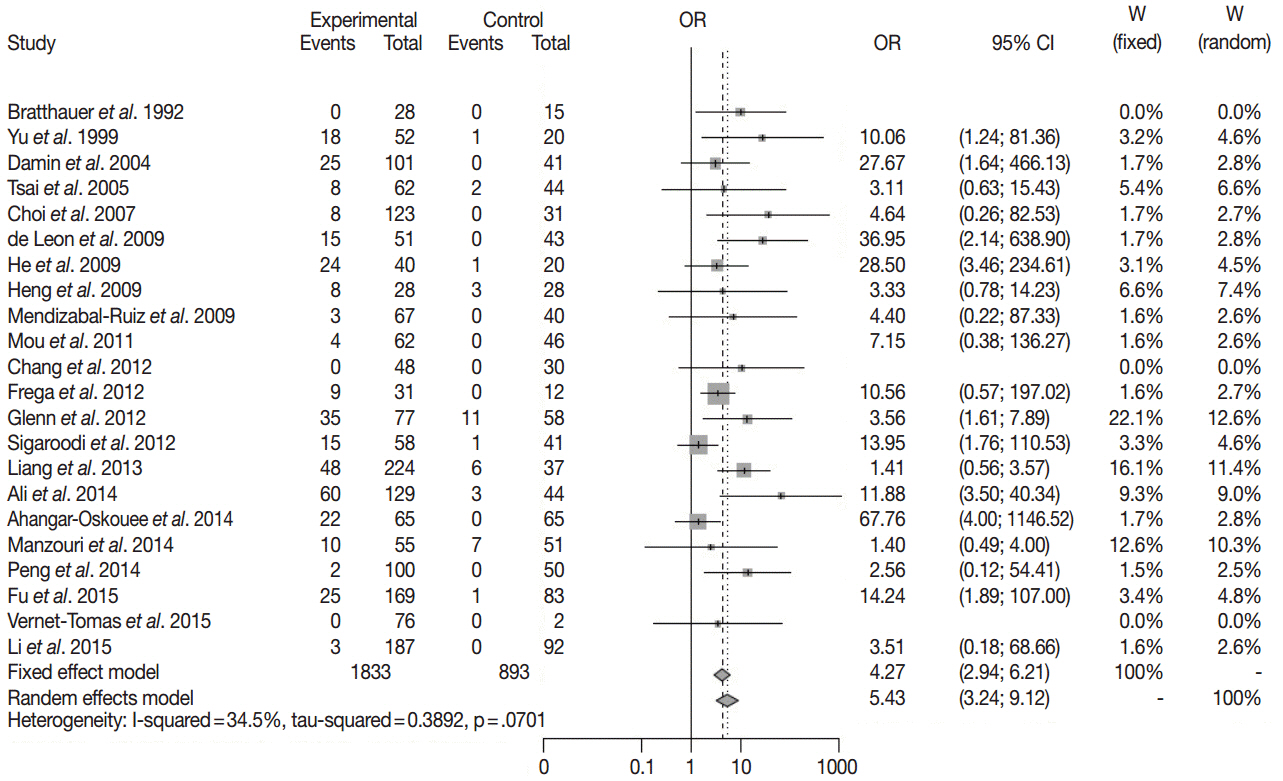
The overall odds ratio between breast cancer and HPV infection was 5.43 (95% confidence interval, 3.24 to 9.12) with I2 = 34.5% in meta-analysis of published studies with case-control setting and it was statistically significant. HPV was detected in 22 cases of breast cancers (17.9%) and two cases of intaductal papillomas (22.2%). However, these cases had weak positivity.
CONCLUSIONS
These results failed to serve as significant evidence to support the relationship between HPV and breast cancer. Further study with larger epidemiologic population is merited to determine the relationship between HPV and breast cancer.
MeSH Terms
Figure
Reference
-
1. Cobos C, Figueroa JA, Mirandola L, et al. The role of human papilloma virus (HPV) infection in non-anogenital cancer and the promise of immunotherapy: a review. Int Rev Immunol. 2014; 33:383–401.
Article2. Wang T, Chang P, Wang L, et al. The role of human papillomavirus infection in breast cancer. Med Oncol. 2012; 29:48–55.
Article3. Lewis A, Kang R, Levine A, Maghami E. The new face of head and neck cancer: the HPV epidemic. Oncology (Williston Park). 2015; 29:616–26.4. Alibek K, Kakpenova A, Mussabekova A, Sypabekova M, Karatayeva N. Role of viruses in the development of breast cancer. Infect Agent Cancer. 2013; 8:32.
Article5. Yu Y, Morimoto T, Sasa M, et al. HPV33 DNA in premalignant and malignant breast lesions in Chinese and Japanese populations. Anticancer Res. 1999; 19:5057–61.6. Hennig EM, Suo Z, Thoresen S, Holm R, Kvinnsland S, Nesland JM. Human papillomavirus 16 in breast cancer of women treated for high grade cervical intraepithelial neoplasia (CIN III). Breast Cancer Res Treat. 1999; 53:121–35.
Article7. Damin AP, Karam R, Zettler CG, Caleffi M, Alexandre CO. Evidence for an association of human papillomavirus and breast carcinomas. Breast Cancer Res Treat. 2004; 84:131–7.
Article8. Tsai JH, Tsai CH, Cheng MH, Lin SJ, Xu FL, Yang CC. Association of viral factors with non-familial breast cancer in Taiwan by comparison with non-cancerous, fibroadenoma, and thyroid tumor tissues. J Med Virol. 2005; 75:276–81.
Article9. Choi YL, Cho EY, Kim JH, et al. Detection of human papillomavirus DNA by DNA chip in breast carcinomas of Korean women. Tumour Biol. 2007; 28:327–32.
Article10. Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel AD, Al Moustafa AE. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer. 2008; 99:404–7.
Article11. de Leon DC, Montiel DP, Nemcova J, et al. Human papillomavirus (HPV) in breast tumors: prevalence in a group of Mexican patients. BMC Cancer. 2009; 9:26.
Article12. He Q, Zhang SQ, Chu YL, Jia XL, Wang XL. The correlations between HPV16 infection and expressions of c-erbB-2 and bcl-2 in breast carcinoma. Mol Biol Rep. 2009; 36:807–12.
Article13. Heng B, Glenn WK, Ye Y, et al. Human papilloma virus is associated with breast cancer. Br J Cancer. 2009; 101:1345–50.
Article14. Mendizabal-Ruiz AP, Morales JA, Ramirez-Jirano LJ, Padilla-Rosas M, Moran-Moguel MC, Montoya-Fuentes H. Low frequency of human papillomavirus DNA in breast cancer tissue. Breast Cancer Res Treat. 2009; 114:189–94.
Article15. Mou X, Chen L, Liu F, et al. Low prevalence of human papillomavirus (HPV) in Chinese patients with breast cancer. J Int Med Res. 2011; 39:1636–44.
Article16. Frega A, Lorenzon L, Bononi M, et al. Evaluation of E6 and E7 mRNA expression in HPV DNA positive breast cancer. Eur J Gynaecol Oncol. 2012; 33:164–7.17. Glenn WK, Heng B, Delprado W, Iacopetta B, Whitaker NJ, Lawson JS. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS One. 2012; 7:e48788.
Article18. Sigaroodi A, Nadji SA, Naghshvar F, Nategh R, Emami H, Velayati AA. Human papillomavirus is associated with breast cancer in the north part of Iran. ScientificWorldJournal. 2012; 2012:837191.
Article19. Liang W, Wang J, Wang C, et al. Detection of high-risk human papillomaviruses in fresh breast cancer samples using the hybrid capture 2 assay. J Med Virol. 2013; 85:2087–92.
Article20. Pereira Suarez AL, Lorenzetti MA, Gonzalez Lucano R, et al. Presence of human papilloma virus in a series of breast carcinoma from Argentina. PLoS One. 2013; 8:e61613.
Article21. Herrera-Goepfert R, Vela-Chavez T, Carrillo-Garcia A, et al. High-risk human papillomavirus (HPV) DNA sequences in metaplastic breast carcinomas of Mexican women. BMC Cancer. 2013; 13:445.
Article22. Francis IM, Al-Ayadhy B, Al-Awadhi S, Kapila K, Al-Mulla F. Prevalence and correlation of human papilloma virus and its types with prognostic markers in patients with invasive ductal carcinoma of the breast in kuwait. Sultan Qaboos Univ Med J. 2013; 13:527–33.
Article23. Ali SH, Al-Alwan NA, Al-Alwany SH. Detection and genotyping of human papillomavirus in breast cancer tissues from Iraqi patients. East Mediterr Health J. 2014; 20:372–7.
Article24. Ahangar-Oskouee M, Shahmahmoodi S, Jalilvand S, et al. No detection of ‘high-risk’ human papillomaviruses in a group of Iranian women with breast cancer. Asian Pac J Cancer Prev. 2014; 15:4061–5.
Article25. Manzouri L, Salehi R, Shariatpanahi S, Rezaie P. Prevalence of human papilloma virus among women with breast cancer since 2005-2009 in Isfahan. Adv Biomed Res. 2014; 3:75.
Article26. Peng J, Wang T, Zhu H, et al. Multiplex PCR/mass spectrometry screening of biological carcinogenic agents in human mammary tumors. J Clin Virol. 2014; 61:255–9.
Article27. Piana AF, Sotgiu G, Muroni MR, Cossu-Rocca P, Castiglia P, De Miglio MR. HPV infection and triple-negative breast cancers: an Italian case-control study. Virol J. 2014; 11:190.
Article28. Li J, Ding J, Zhai K. Detection of human papillomavirus DNA in patients with breast tumor in China. PLoS One. 2015; 10:e0136050.
Article29. Fernandes A, Bianchi G, Feltri AP, Perez M, Correnti M. Presence of human papillomavirus in breast cancer and its association with prognostic factors. Ecancermedicalscience. 2015; 9:548.
Article30. Lindel K, Forster A, Altermatt HJ, Greiner R, Gruber G. Breast cancer and human papillomavirus (HPV) infection: no evidence of a viral etiology in a group of Swiss women. Breast. 2007; 16:172–7.
Article31. Hedau S, Kumar U, Hussain S, et al. Breast cancer and human papillomavirus infection: no evidence of HPV etiology of breast cancer in Indian women. BMC Cancer. 2011; 11:27.
Article32. Kwong A, Leung CP, Shin VY, Ng EK. No evidence of human papillomavirus in patients with breast cancer in Hong Kong, Southern China. ISRN Virol. 2013; 2013:546503.
Article33. Vernet-Tomas M, Mena M, Alemany L, et al. Human papillomavirus and breast cancer: no evidence of association in a Spanish set of cases. Anticancer Res. 2015; 35:851–6.34. Bratthauer GL, Tavassoli FA, O’Leary TJ. Etiology of breast carcinoma: no apparent role for papillomavirus types 6/11/16/18. Pathol Res Pract. 1992; 188:384–6.
Article35. Chang P, Wang T, Yao Q, et al. Absence of human papillomavirus in patients with breast cancer in north-west China. Med Oncol. 2012; 29:521–5.
Article36. Fu L, Wang D, Shah W, Wang Y, Zhang G, He J. Association of human papillomavirus type 58 with breast cancer in Shaanxi province of China. J Med Virol. 2015; 87:1034–40.
Article37. Beckmann MW, Niederacher D, Schnurch HG, Gusterson BA, Bender HG. Multistep carcinogenesis of breast cancer and tumour heterogeneity. J Mol Med (Berl). 1997; 75:429–39.
Article38. Ohba K, Ichiyama K, Yajima M, et al. In vivo and in vitro studies suggest a possible involvement of HPV infection in the early stage of breast carcinogenesis via APOBEC3B induction. PLoS One. 2014; 9:e97787.39. Khan NA, Castillo A, Koriyama C, et al. Human papillomavirus detected in female breast carcinomas in Japan. Br J Cancer. 2008; 99:408–14.
Article40. Kan CY, Iacopetta BJ, Lawson JS, Whitaker NJ. Identification of human papillomavirus DNA gene sequences in human breast cancer. Br J Cancer. 2005; 93:946–8.
Article41. de Villiers EM, Sandstrom RE, zur Hausen H, Buck CE. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res. 2005; 7:R1–11.
Article42. Lv YR, Wang JL, Zhang K, et al. Human papilloma viruses (HPVs) no co-existence in breast cancer and cervical cells in the same patient. Chin J Physiol. 2014; 57:105–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Human Papillomavirus in Recurrent Conjunctival Papilloma
- Identification of the types of human papillomavirus in condylomata acuminata using polymerase chain reaction
- Detection of Genital Human Papilloma Viruses Using PCR
- Association of Human Papillomavirus with Human Colorectal Cancer
- Detection of Human Papillomavirus in Uterine Cervical Cancer Tissues by Polymerase Chain Reaction, In Situ Hybridization and Polymerase Chain Reaction In Situ Techniques